Published online Oct 1, 2004. doi: 10.3748/wjg.v10.i19.2864
Revised: April 22, 2004
Accepted: April 29, 2004
Published online: October 1, 2004
AIM: To evaluate the clinical value of plasma apolipoprotein A-I (Apo A-I) as a marker of fibrosis in children with chronic hepatitis B (CHB).
METHODS: Liver biopsy specimens from 49 children with CHB were evaluated by using Knodell index. Plasma Apo A-I level was measured after 12-h fasting. Student’s t test, Spearman’s correlation test and receptor-operating characteristic (ROC) curve were used for statistical evaluation.
RESULTS: Mean Apo A-I level of the patients was not different from that of controls (P > 0.05). Six (8.7%) children had fibrosis score of more than 2 (severe fibrosis). No difference in the level of mean plasma Apo A-I was found among children with and without severe fibrosis (P > 0.05). No correlation between Apo A-I level and fibrosis scores was found (P > 0.05). The area under the ROC curve was 0.407 ± 0.146 (P > 0.05).
CONCLUSION: Severe fibrosis is not common in children with CHB and plasma Apo A-I level is not a reliable indicator of fibrosis.
- Citation: Selimoglu MA, Yagcl RV, Yüce G. Low plasma apolipoprotein A-I level is not a reliable marker of fibrosis in children with chronic hepatitis B. World J Gastroenterol 2004; 10(19): 2864-2866
- URL: https://www.wjgnet.com/1007-9327/full/v10/i19/2864.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i19.2864
Apolipoprotein A-I (Apo A-I) is produced mainly by hepatocytes and its level varies according to the degree of liver fibrosis[1-4]. In our previous study, we demonstrated the inverse correlation of Apo A-I with the Child-Pugh score[3] in children with cirrhosis. Data of Apo A-I levels in chronic hepatitis were mainly from studies performed in adults with hepatitis C[5,6]. A recent study showed that serum Apo A-I level was inversely correlated with liver fibrosis in adult patients with chronic hepatitis B (CHB)[7].
Liver biopsy is an invasive procedure especially for children. It is well known that serum alanine transaminase (ALT) is a good indicator of portal inflammation in chronic hepatitis[8], but a reliable biochemical fibrosis index is not available for children. The aim of this study was to determine if low plasma Apo A-I level was a reliable indicator of fibrosis in childhood CHB.
Forty-nine hepatitis Be antigen (HBeAg) positive children (13 females and 36 males) with pathology verified chronic HBV infection were included in this study. Their mean age was 9.1 ± 4.1 years (3 to 18 years). Patients were excluded if they had hepatitis D or C or HIV infection. No patient had evidence of decompensated liver diseases or autoimmune hepatitis. Parents of the patients were required to give written informed consent before entering the study.
Pretreatment liver biopsy specimens were obtained from all patients and scored with the use of the Knodell index[9], which grades the histological activity of hepatitis on a scale from 0 to 22, with higher scores indicating more severe abnormalities. The overall Knodell score is the sum of the scores for periportal/ bridging necrosis (0 to 10), intralobular degeneration and focal necrosis (0 to 4), portal inflammation (0 to 4), and fibrosis (0 to 4). Before the patients entered the study, blood samples were taken for the test of blood cell counts, serum alanine aminotransferase (ALT), Apo A-I, hepatitis B surface antigen (HBsAg), HBeAg, antibody to HBeAg, antibody to HBsAg and HBV DNA.
Plasma Apo A-I levels of 20 gender- and age-matched healthy children were used as controls. Student’s t test, Spearmen’s correlation test and ROC curve were used for statistical analysis.
Pre treatment ALT, Apo A-I, hepatic activity index (HAI), portal fibrosis, inflammation and necrosis scores, and HBV DNA levels of the children are shown in Table 1. Mean plasma Apo A-I level of 49 children with CHB was not different from that of controls (39.3 ± 5.8 μmol/L vs 40.71 ± 4.8 μmol/L, P > 0.05).
| Parameter | mean ± SD | Range |
| ALT (IU/l) | 80 ± 80 | 21-396 |
| Apo A-I (μmol/L) | 39.3 ± 5.8 | 29.2-57.5 |
| Hepatic activity index | 7.0 ± 3.0 | 3-17 |
| Portal fibrosis | 1.4 ± 0.9 | 0-4 |
| Portal inflammation | 2.0 ± 1.2 | 0-4 |
| Focal necrosis | 1.6 ± 0.9 | 0-4 |
| Bridging necrosis | 2.0 ± 1.6 | 0-6 |
| HBV DNA (ng/L) | 1537 ± 712 | 118-3111 |
Children with CHB were divided into 2 groups according to their fibrosis stage: group 1, whose portal fibrosis scores were 0, 1 or 2; group 2, whose portal fibrosis scores were 3 or 4. In groups 1 and 2, the mean Apo A-I levels were 39.6 ± 5.6 μmol/L and 36.9 ± 6.8 μmol/L, respectively (P > 0.05). Only 6 (8.7%) children had a fibrosis score of more than 2.
No correlation among Apo A-I and HAI, portal inflammation, portal fibrosis, focal necrosis, bridging necrosis scores was found (P > 0.05). A positive correlation between ALT and portal inflam-mation scores and between ALT and HAI was found (P < 0.05 and P < 0.01, respectively).
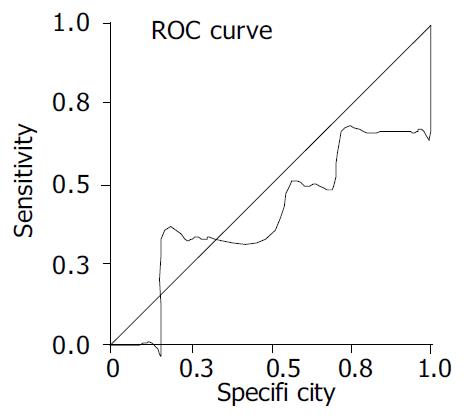
The area under the ROC curve of Apo A-I was 0.407 ± 0.146 (P > 0.05, Figure 1).
Fibronectin, a major liver extracellular matrix component, can interact with Apo A-I both by downregulating its mRNA level in liver cells and by binding to this molecule after its secretion in the extracellular space[1,2]. Since fibronectin is the first matrix component to be produced in excess and deposited in liver fibrosis, it has been thought to be involved in the decrease in Apo A-I in alcoholic patients with liver fibrosis and cirrhosis[1,2].
Because liver biopsy is an invasive method with a 1/10000 mortality rate, noninvasive serum markers of liver fibrosis are under investigation[10]. Furthermore, although histological analysis is considered to be the gold standard for the diagnosis of extensive fibrosis and cirrhosis, the rate of false-negative results was approximately 15%-20%[10]. Clinical investigations on fibrosis markers were generally focused on hepatitis C rather than hepatitis B[5,6]. One of the few studies performed of patients with CHB was the study Huang et al[7]. They investigated the value of serum biochemical markers in the diagnosis of liver fibrosis of patients with hepatitis B and observed that levels of ALT, total bilirubin, alpha 2-macroglobulin, GGT and Apo A-I were signi-ficantly correlated with the clinical staging of liver fibrosis, and concluded that a combined assessment of these indices might help obtain an accurate diagnosis of liver cirrhosis with less need of pathological biopsy in this population[7].
Besides noninvasive biochemical markers, some studies strongly recommended using some historical features such as sex, age at biopsy and alcohol consumption as independent predictors of fibrosis in chronic hepatitis C adults[5]. They demonstrated that a simple index including age, sex, and five biochemical markers could accurately predict significant hepatitis C-related fibrosis[5].
Teare et al[11] compared aminoterminal type III procollagen propeptide (PIIIP) with the PGA index, which combines the prothrombin time, gamma-glutamyl transpeptidase activity, and serum Apo A-I concentration, in predicting liver fibrosis in patients with alcoholic liver disease, primary biliary cirrhosis, and CHB. They stated that PGA and PIIIP values were increased in all patients with CHB in comparison with controls. For the detection of cirrhosis they found that PGA was 91% sensitive and 81% specific[11]. Similarly Ding et al[12] evaluated the diagnostic value of serum liver fibrosis markers by analyzing the correlation between liver fibrosis stage in patients with CHB and serum liver fibrosis markers, such as hyaluronate, type III procollagen, laminin, and type IV collagen in 278 patients. They found that liver fibrosis stage was correlated to inflammation degree and that serum hyaluronate, type III procollagen, laminin, and type IV collagen could reflect the state of liver fibrosis[12].
The difference between mean Apo A-I level of children with CHB and controls was not significant (39.3 ± 5.8 μmol/L vs 40.71 ± 4.8 μmol/L). In 2002, we reported similar figures for children with chronic hepatitis, most of them had CHB[4]. However, Norton et al[13] reported that Apo A-I and apo C-III steady-state mRNA levels were suppressed by HBV replication and/or gene expression. Other studies revealed that the levels of Apo A-I and Apo A-II were reduced during acute hepatitis, when virus levels were high, and normalized after viral clearance[14,15]. Similar results were reported for chronic HBV infection. Reduced levels of circulating lipoproteins and apolipoproteins have been correlated with increased viral replication[16-18]. In our study no correlation between HBV DNA and Apo A-I levels was detected.
Of the 49 children, only 8.7% had significant fibrosis according to Knodell index (> 2), suggesting that fibrosis is not prevalent in this age group. Although the mean plasma Apo A-I level was lower in this small population compared to children with mild/no fibrosis (36.9 ± 6.8 μmol/L vs 39.6 ± 5.6 μmol/L), statistical significance was not detected.
Recently it has been recommended to use ROC curves in determ-ination of the indices of hepatic fibrosis in clinical practice[19,20]. In this study, plasma Apo A-I level as a fibrosis marker was measured in children with CHB and compared with Knodell scores to determine if it had any clinical value as a marker of fibrosis. In conclusion, severe fibrosis and Knodell score higher than 2, are not common in CHB children and plasma Apo A-I level is not a reliable indicator of fibrosis.
Edited by Chen WW and Wang XL Proofread by Xu FM
| 1. | Paradis V, Laurent A, Mathurin P, Poynard T, Vidaud D, Vidaud M, Bedossa P. Role of liver extracellular matrix in transcriptional and post-transcriptional regulation of apolipoprotein A-I by hepatocytes. Cell Mol Biol (Noisy-le-grand). 1996;42:525-534. [PubMed] |
| 2. | Paradis V, Mathurin P, Ratziu V, Poynard T, Bedossa P. Binding of apolipoprotein A-I and acetaldehyde-modified apolipoprotein A-I to liver extracellular matrix. Hepatology. 1996;23:1232-1238. [PubMed] [DOI] [Full Text] |
| 3. | Selimoğlu MA, Aydoğdu S, Yağci RV. Low plasma apolipoprotein A-I level: new prognostic criterion in childhood cirrhosis. Turk J Pediatr. 2001;43:307-311. [PubMed] |
| 4. | Selimoglu MA, Aydogdu S, Yagci RV. Lipid parameters in childhood cirrhosis and chronic liver disease. Pediatr Int. 2002;44:400-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Myers RP, Ratziu V, Imbert-Bismut F, Charlotte F, Poynard T. Biochemical markers of liver fibrosis: a comparison with historical features in patients with chronic hepatitis C. Am J Gastroenterol. 2002;97:2419-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Poynard T, Imbert-Bismut F, Ratziu V, Chevret S, Jardel C, Moussalli J, Messous D, Degos F. Biochemical markers of liver fibrosis in patients infected by hepatitis C virus: longitudinal validation in a randomized trial. J Viral Hepat. 2002;9:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Huang W, Gong FY. [Diagnostic value of serum biochemical markers for liver fibrosis in patients with hepatitis B virus]. Diyi Junyi Daxue Xuebao. 2002;22:1034-1036. [PubMed] |
| 8. | Tang TJ, Kwekkeboom J, Laman JD, Niesters HG, Zondervan PE, de Man RA, Schalm SW, Janssen HL. The role of intrahepatic immune effector cells in inflammatory liver injury and viral control during chronic hepatitis B infection. J Viral Hepat. 2003;10:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Desmet VJ. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis [Hepatology 1981; 1: 431-435]. J Hepatol. 2003;38:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2558] [Cited by in RCA: 2509] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 10. | Cadranel JF, Mathurin P. Prothrombin index decrease: a useful and reliable marker of extensive fibrosis. Eur J Gastroenterol Hepatol. 2002;14:1057-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Teare JP, Sherman D, Greenfield SM, Simpson J, Bray G, Catterall AP, Murray-Lyon IM, Peters TJ, Williams R, Thompson RP. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet. 1993;342:895-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Ding H, Chen Y, Feng X, Liu D, Wu A, Zhang L. [Correlation between liver fibrosis stage and serum liver fibrosis markers in patients with chronic hepatitis B]. Zhonghua Ganzangbing Zazhi. 2001;9:78-80. [PubMed] |
| 13. | Norton PA, Gong Q, Mehta AS, Lu X, Block TM. Hepatitis B virus-mediated changes of apolipoprotein mRNA abundance in cultured hepatoma cells. J Virol. 2003;77:5503-5506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Vergani C, Trovato G, Delu A, Pietrogrande M, Dioguardi N. Serum total lipids, lipoprotein cholesterol, and apolipoprotein A in acute viral hepatitis and chronic liver disease. J Clin Pathol. 1978;31:772-778. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Vergani C, Trovato G, Pietrogrande M, Crocchiolo P, Dioguardi N. [Behavior of total lipids, cholesterol, lipoproteins and apolipoprotein A in the blood of subjects with acute hepatitis and chronic hepatopathy]. Minerva Med. 1978;69:2081-2094. [PubMed] |
| 16. | Chen Z, Keech A, Collins R, Slavin B, Chen J, Campbell TC, Peto R. Prolonged infection with hepatitis B virus and association between low blood cholesterol concentration and liver cancer. BMJ. 1993;306:890-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Cordova C, Musca A, Violi F, Alessandri C, Iuliano L. Apolipoproteins A-I, A-II and B in chronic active hepatitis and in liver cirrhotic patients. Clin Chim Acta. 1984;137:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Fehér J, Romics L, Jakab L, Fehér E, Szilvási I, Papp G. Serum lipids and lipoproteins in chronic liver disease. Acta Med Acad Sci Hung. 1976;33:217-223. [PubMed] |
| 19. | Zheng M, Cai WM, Weng HL, Liu RH. ROC curves in evaluation of serum fibrosis indices for hepatic fibrosis. World J Gastroenterol. 2002;8:1073-1076. [PubMed] |
| 20. | Walsh KM, Timms P, Campbell S, MacSween RN, Morris AJ. Plasma levels of matrix metalloproteinase-2 (MMP-2) and tissue inhibitors of metalloproteinases -1 and -2 (TIMP-1 and TIMP-2) as noninvasive markers of liver disease in chronic hepatitis C: comparison using ROC analysis. Dig Dis Sci. 1999;44:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |