Published online Sep 1, 2004. doi: 10.3748/wjg.v10.i17.2571
Revised: January 4, 2004
Accepted: January 8, 2004
Published online: September 1, 2004
AIM: To develop a novel process for production of HAV in Vero cells grown on microcarriers in a bioreactor.
METHODS: Vero cells infected with HAV strain W were seeded at an initial density of 1 × 105 cells/mL into a 7-L bioreactor containing Cytodex-I microcarriers. During the stage of cell proliferation, the following conditions were applied: pH7.2 ± 0.2, temperature 37 ± 0.2 °C, dissolved oxygen 40% of air saturation and agitation rate 40 r/min . After the stage of virus culture started, the culture conditions were altered to pH7.2 ± 0.2, temperature 35 ± 0.2 °C, dissolved oxygen 25% of air saturation, agitation rate 50 r/min and perfusion of fresh medium at a flux of 20 mL/h. During the course of fermentation, cell density, HAV antigen titre, glucose, lactate and ammonia levels were monitored. A control experiment using conventional static culture was conducted in the T150 flask.
RESULTS: After a 28-d cultivation, cell density increased to 14.0 × 105 cells/mL in the bioreactor, 5.6 × 109 viable cells and 4000 mL virus suspension with a titre of 1:64 were harvested. The viral antigen output per cell unit in the bioreactor was 3-fold higher than that in the T150 flask. Meanwhile the metabolic mode of Vero cells did not change after the infection with HAV strain W.
CONCLUSION: The process for production of HAV in Vero cells grown on microcarriers in a bioreactor is a novel, efficient and practical way to obtain virus antigen for vaccine purpose. This approach produces more cells and HAV antigen than the conventional static culture. With futher improvement, it is possible to be used for the production of hepatitis A vaccine.
- Citation: Sun MB, Jiang YJ, Li WD, Li PZ, Li GL, Jiang SD, Liao GY. A novel process for production of hepatitis A virus in Vero cells grown on microcarriers in bioreactor. World J Gastroenterol 2004; 10(17): 2571-2573
- URL: https://www.wjgnet.com/1007-9327/full/v10/i17/2571.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i17.2571
Hepatitis A continues to be one of the most frequently reported infectious diseases with a worldwide distribution, and the continued occurrence of extensive community wide outbreaks indicates that hepatitis A remains a major public health problem. The availability of hepatitis A vaccine provides the opportunity to substantially lower disease incidence and potentially eliminate infection[1,2]. Several inactivated and live-attenuated hepatitis A vaccines have been developed. Due to the high protective efficacy, immunogenicity and safety, the inactivated vaccines are more favored[3-6]. At present several inactivated hepatitis A vaccines such as HAVRIXR, VAQTAR, AVAXIMR and EPAXALR are commercially available[7-9]. Inactivated hepatitis A vaccine is prepared by methods similar to those used for inactivated poliovirus vaccines. Cell-culture-adapted virus is propagated in human fibroblast cells, usually in MRC-5 cells, purified from cell lysates by ultrafiltration and exclusion gel chromatography or other methods, inactivated with formalin, and absorbed to an aluminum hydroxide adjuvant. Compared with the production of live- attenuated vaccines, much larger quantities of HAV antigen are required for the production of inactivated vaccines. Thus the production of inactivated hepatitis A vaccines is usually hampered by the difficulty of preparation of large amounts of virus antigens in the conventional static flask or roller bottle cultures. The technique of microcarrier cell culture was first developed in the late 1960 s and has been used successfully for the production of rabies and poliomyelitis vaccines. One definite advantage of microcarrier cell culture is the ability to offer a high product output, which may help to solve the shortage of virus antigens for production of inactivated hepatitis A vaccines[10-13].
As mentioned above, most hepatitis A vaccines are commonly prepared by cultivation of HAV in human fibroblast cells including MRC-5 cells. No vaccine is produced by microcarrier technology due to the unsuitability of growth for MRC-5 cells on microcarriers. For HAV production in a microcarrier system it is necessary to establish suitable cells on microcarriers. Vero cells can grow well on microcarriers. But HAV is very difficult to adapt to grow in Vero cells. There are few reports about the isolation and adaptation of HAV in Vero cells. Until recently, no report about the production of hepatitis A vaccines in Vero cells grown on microcarriers in bioreactor is available. To verify the feasibility of this approach, HAV strain W was isolated and adapted in Vero cells in our previous work. This study described the production of HAV strain W in Vero cells grown on Cytodex-I microcarriers in a 7-L bioreactor.
A 7-L cell bioreactor (Applikon. Co., Holland) was used. Its work volume is 4 L. The value of dissolved oxygen was regulated by importing air mixture with different ratios of O2, N2, CO2 and air. The value of pH was controlled by addition of NaHCO3.
Cytodex-I microcarrier (Pharmacia Fine Chemicals, Uppsala, Sweden) was used.
Cells were grown in minimum essential medium (MEM; Gibco, Glasgow, UK) supplemented with 100 U/mL penicillin, 10 mmol/L of glucose, 5 mmol/L of glutamine and 100 mL/L bovine calf serum. In both virus culture medium and the feeding medium, bovine calf serum was reduced to 2%.
Vero cell line was obtained from ATCC and preserved at our laboratory. Vero cells were used between passages 142 and 146 in this study. HAV strain W was isolated and adapted in Vero cells at our laboratory. The antigen titre of HAV used for inoculation was 1:128.
Vero cells were seeded into the T150 flasks at the concentration allowing for the formation of a confluent monolayer in 4-5 d. The monolayer cells were trypinsized and collected to make cell suspension in a Bellco agitation bottle. The cell suspension was inoculated with HAV strain W and incubated at 35 °C with a stirring of 30 rpm for 6 h.
Bioreactor culture was divided into two stages including cell proliferation and virus culture. Infected Vero cells were seeded into the 7-L bioreactor at an initial density of 1 × 105 cells/mL. During the stage of cell proliferation, the following conditions were applied: pH7.2 ± 0.2, temperature 37 ± 0.2 °C, dissolved oxygen 40% of air saturation and agitation rate 40 r/min. After the cell density reached to 1 × 106 cells/mL, stage of virus culture started and the used medium was replaced with maintenance medium. The culture conditions were altered to: pH7.2 ± 0.2, temperature 35 ± 0.2 °C, dissolved oxygen 25% of air saturation and agitation rate 50 r/min. Meanwhile, perfusion was started and the perfusion rate was modulated to 20 mL/h. After a 28-d culture, infected cells were trypinsized and collected for the harvest of HAV antigen.
In a further study, M199 medium was used instead of MEM during virus culture. A circulating equipment was used to increase the dissolved oxygen in the feeding medium.
Infected Vero cells were seeded at an initial density of 1 × 105 cells/mL into the T150 flask containing 150 mL MEM medium. The cultures were incubated at 35 °C and the medium was replaced each week. After a 28-d culture, infected cells were trypinsized and collected for the harvest of HAV antigen.
Samples taken from the cultures in the bioreactor were centrifuged at 1500 r/min for 10 min. The supernatants were stored at -20 °C until tested. Glucose, ammonia and lactate concentrations were monitored by enzymatic assays, using specific assay kits (16-UV, 171-UV and 826-UV for glucose, ammonia and lactate respectively; Sigma, St. Louis, USA).
Harvested cells were applied to three cycles of freezing and thawing following ultrasonication. After centrifugation at 1500 r/min for 10 min, HAV antigen titre in the supernatant was determined by ELISA.
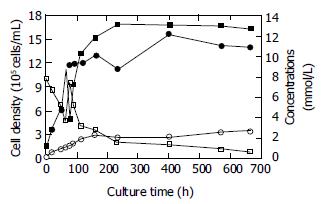
Seventy-eight hours after the seeding, infected Vero cell density increased from 1 × 105 cells/mL to 11.17 × 105 cells/mL. At this point, the mode of bioreactor culture was switched from cell proliferation to virus culture. During the stage of virus culture, the cell density was kept above 1 × 106 cells/mL, reaching a maximum of 15.60 × 105 cells/mL at 402 h. It achieved 14.0 × 105 cells/mL at the end of virus culture, indicating a 14-fold increase over the initial cell density. The initial concentration of glucose was 7.8 mmol/L at the beginning of cell proliferation stage. As the cell density increased, the concentration declined. The rate of declination gradually became slow as a result of the feeding of fresh medium. The residual glucose concentration was 0.63 mmol/L at the end of virus culture. The initial concentrations of lactate and ammonia were 1.27 mmol/L and 0.18 mmol/L respectively at the beginning of cell proliferation and increased along with the increasing of cell density. The lactate level increased from 3.87 mmol/L to 12.68 mmol/L and ammonia level from 1.19 mmol/L to 2.68 mmol/L during the stage of virus culture. The rate of accumulation also became slow due to the feeding of fresh medium at the end of virus culture. Figure 1 shows the variations of cell density, glucose, lactate, and ammonia levels.
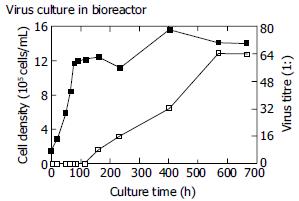
No HAV antigen was detected during the stage of cell proliferation and at the early stage of virus culture. At 160 h, HAV antigen was first detected with a titre of 1:8 and achieved a maximum of 1:64 at 570 h (Figure 2). Four thousand mililitres of virus suspension with a titre of 1:64 was obtained at the end of virus culture.
After a 28-d cultivation, 4 × 107 viable cells and 5 mL virus suspension with a titre of 1:128 were obtained from a T150 flask.
MEM medium was replaced by M199 medium, which contained richer nutriments and was more suitable for virus growth in cells. A circulating equipment was added to increase the dissolved oxygen in the feeding medium. With these modifications, antigen titre achieved 1:128 in the harvested suspension.
In this study the growth kinetics and metabolic properties of Vero cells infected with HAV strain W were investigated. The results were similar to those of uninfected Vero cells in our previous work (Data not shown) and other studies[14,15]. This indicated that the metabolic mode of Vero cells did not change after infection with HAV strain W.
The yield of viable cells was 5.6 × 109 in the bioreactor whereas 4 × 107 in the T150 flask. The former was 140-fold higher than the latter. The yield of HAV antigens was 4000 mL with a titre of 1:64 in the bioreactor and equaled to the yield from 400 T150 flasks. Furthermore, per 105 cells produced 1 mL HAV antigens with a titre of 1:4.57 in a bioreactor compared with only a titre of 1:1.6 in the T150 flask, that is the antigen output per cell unit in the bioreactor was 3-fold higher than that in the T150 flask. The findings mentioned above demonstrated this process for the production of HAV in Vero cells grown on microcarriers in a bioreactor could provide much higher yields of viable cells and virus antigens than that in conventional static culture. The antigen output per cell unit was also apparently higher. The technique of microcarrier culture in a bioreactor could offer numerous advantages over traditional techniques, such as a high ratio of surface area to volume, an efficient monitoring and control of key process parameters including temperature, pHand dissolved oxygen. Moreover, the culture substrate conditions could be controlled exactly and regulated in batch or fed-batch operation. The optimal metabolism and physiological status of cells could be achieved through the feeding of fresh medium and removal of toxic metabolites. Thus a high cell density and product output rate could be easily achieved[15-18].
In conclusion, the process for production of HAV in Vero cells grown on microcarriers in a bioreactor is a novel, efficient and practical way to obtain virus antigens for vaccine purpose. This approach produces more cells and HAV antigens than the conventional static culture. The optimal growth conditions for Vero cells and HAV strain W in a bioreactor are being further investigated to enhance the yield of virus antigens.
Edited by Zhang JZ and Wang XL Proofread by Xu FM
| 1. | Zamir C, Rishpon S, Zamir D, Leventhal A, Rimon N, Ben-Porath E. Control of a community-wide outbreak of hepatitis A by mass vaccination with inactivated hepatitis A vaccine. Eur J Clin Microbiol Infect Dis. 2001;20:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Van Damme P, Banatvala J, Fay O, Iwarson S, McMahon B, Van Herck K, Shouval D, Bonanni P, Connor B, Cooksley G. Hepatitis A booster vaccination: is there a need. Lancet. 2003;362:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Demicheli V, Tiberti D. The effectiveness and safety of hepatitis A vaccine: a systematic review. Vaccine. 2003;21:2242-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Xu Z, Wang X, Li R, Meng Z, Zhang Y, Gong J, Ma J, Li Y, Zhao S, Li Y. [Immunogenicity and efficacy of two live attenuated hepatitis A vaccines (H (2) strains and LA-1 strains)]. Zhonghua Yixue Zazhi. 2002;82:678-681. [PubMed] |
| 5. | Ren A, Ma J, Feng F. [Safety and immunogenicity of a new inactivated hepatitis A vaccine]. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2001;15:357-359. [PubMed] |
| 6. | Ren YH, Chen JT, Wu WT, Gong XJ, Zhang YC, Xue WH, Ren YF, Han LJ, Kang WX, Li SP. [The study on the 0, 12 month vaccination schedule' of Healive inactivated hepatitis A vaccine in children]. Zhonghua Liuxing Bingxue Zazhi. 2003;24:1013-1015. [PubMed] |
| 7. | Huang G, Wan Z, Li R. [The investigation on the safety and immunogenicity of inactived hepatitis A vaccine AVAXIM]. Zhonghua Liuxingbingxue Zazhi. 2000;21:287-288. [PubMed] |
| 8. | Linglöf T, van Hattum J, Kaplan KM, Corrigan J, Duval I, Jensen E, Kuter B. An open study of subcutaneous administration of inactivated hepatitis A vaccine (VAQTA) in adults: safety, tolerability, and immunogenicity. Vaccine. 2001;19:3968-3971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Usonis V, Bakasénas V, Valentelis R, Katiliene G, Vidzeniene D, Herzog C. Antibody titres after primary and booster vaccination of infants and young children with a virosomal hepatitis A vaccine (Epaxal). Vaccine. 2003;21:4588-4592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Williams JL, Bruden DA, Cagle HH, McMahon BJ, Negus SE, Christensen CJ, Snowball MM, Bulkow LR, Fox-Leyva LK. Hepatitis A vaccine: immunogenicity following administration of a delayed immunization schedule in infants, children and adults. Vaccine. 2003;21:3208-3211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Franco E, Vitiello G. Vaccination strategies against hepatitis A in southern Europe. Vaccine. 2003;21:696-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Hennessey JP, Oswald CB, Dong Z, Lewis JA, Sitrin RD. Evaluation of the purity of a purified, inactivated hepatitis A vaccine (VAQTA). Vaccine. 1999;17:2830-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Murdoch DL, Goa K, Figgitt DP. Combined hepatitis A and B vaccines: a review of their immunogenicity and tolerability. Drugs. 2003;63:2625-2649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Sun MB, Zhang LJ, Liao GY, Li GL, Jiang SD. Physiology of Vero, CHO and Hybridoma cells cultured in bioreactor. Zhongguo Shengwuzhipinxue Zazhi. 2001;14:213-216. |
| 15. | Guan YH, Kemp RB. Detection of the changing substrate requirements of cultured animal cells by stoichiometric growth equations validated by enthalpy balances. J Biotechnol. 1999;69:95-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Voigt A, Zintl F. Hybridoma cell growth and anti-neuroblastoma monoclonal antibody production in spinner flasks using a protein-free medium with microcarriers. J Biotechnol. 1999;68:213-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Kallel H, Rourou S, Majoul S, Loukil H. A novel process for the production of a veterinary rabies vaccine in BHK-21 cells grown on microcarriers in a 20-l bioreactor. Appl Microbiol Biotechnol. 2003;61:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Dürrschmid M, Landauer K, Simic G, Blüml G, Doblhoff-Dier O. Scalable inoculation strategies for microcarrier-based animal cell bioprocesses. Biotechnol Bioeng. 2003;83:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |