Published online Sep 1, 2004. doi: 10.3748/wjg.v10.i17.2567
Revised: December 4, 2003
Accepted: December 16, 2003
Published online: September 1, 2004
AIM: To investigate the effects of anti-Fas ribozyme on Fas expression and apoptosis in primary cultured mouse hepatocytes.
METHODS: Mouse hepatocytes were isolated by using collagenase irrigation. A hammerhead ribozyme targeting the Fas mRNA was constructed, and transfected into mouse hepatocytes via Effectene. Then Fas expression in mouse hepatocytes was detected by RT-PCR and western blotting. After being treated with anti-Fas antibody (JO2), hepatocytes viability was measured with MTT assay. Caspase-3 proteolytic activity was detected, and cell apoptosis was measured according to Annexin V-FITC apoptosis detection kit.
RESULTS: Fas expressed in primary mouse hepatocytes. Fas expression in hepatocytes transfected with anti-Fas ribozyme was decreased remarkably and correlated with the resistance to Fas-mediated apoptosis as determined by flow cytometry and caspase-3 proteolytic activity.
CONCLUSION: Anti-Fas ribozyme can remarkably decrease the Fas expression in mouse hepatocytes, thus inhibit Fas-mediated apoptosis in hepatocytes. It is suggested that anti-Fas ribozyme could significantly increase the resistance of transplanted hepatocytes to apoptosis and improve the survival of transplanted hepatocytes.
- Citation: Zhang M, He W, Liu F, Zou P, Xiao J, Zhong ZD, Hu ZB. Inhibition of mouse hepatocyte apoptosis via anti-Fas ribozyme. World J Gastroenterol 2004; 10(17): 2567-2570
- URL: https://www.wjgnet.com/1007-9327/full/v10/i17/2567.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i17.2567
Liver transplantation is one of the efficient ways to treat liver function failure and liver necrosis induced by all kinds of diseases[1,2]. But Fas expresses in hepatocytes and Fas-mediated apoptosis significantly impairs graft survival[3-7]. Anti-Fas hammerhead ribozyme was designed and synthesized to target directly at position 596 of the Fas RNA, and cloned into a eukaryotic vector, which was transfected into primary cultured mouse hepatocytes via a vector named Effectene different from usual Lipofectamine reagents[8]. Then the effects of anti-Fas ribozyme on Fas expression and apoptosis of mouse hepatocytes were investigated.
E.coli DH-5α was a kind gift from Department of Immunology, Tongji Medical College. Prokaryotic vector pBSKU6 and green fluorescent protein pEGFPC1 were given as presents by Dr. Kongxinjuan. All restriction endonucleases and T4 DNA ligase were products of Promega Company. Mini plasmid DNA extraction kit, gel DNA purification kit and DL-2000 DNA marker were purchased from TaKaRa Company. Transfection reagent Effectene was purchased from Qiagen Company. Reverse transcriptional kit, dNTP, Taq DNA polymerase were products of MBI Company. Fluorescein isothiocyanate (FITC) -conjugated Annexin V kit was product of Bender Company (purchased from Jingmei Company). FITC-conjugated anti-mouse Fas antibody (JO2) was product of PharMingen Company. Caspase-3 activity detection kit was product of Clontech Company. MTT reagent was purchased from Sigma Company.
GUA triplets located at 596 of the mouse Fas RNA were selected as the cleavage site of ribozyme[9], two small nucleotide sequences complementary to flanks of the cleavage site of target RNA were located before and after hammerhead structure-the conservative core catalytic sequence of ribozyme, which can form typical active cleavage structure through complementation[10]. The cDNAs encoding the anti-Fas ribozyme were composed of two complementary strands each about 50 nt, which were terminated with BamH I and Xba I: a 5’ TCTAGAGATATATAAACTGATGAGTCCGTGAGGACGAAACAAGTGGATCC 3’, b 5’ GGATCCACTTGTTTCGTCCTCACGGACTCATCAGTTTATATATCTCTAGA 3’. All cDNAs were synthesized by Shanghai Shenggong Company. The ribozyme was cloned into the BamH I and Xba I sites of the pBSKU6, which was named as U6-RZ596, and digested and sequenced to be correct. Then this recombinant plasmid was used as templete to get a fragment including U6 promoter and ribozyme by using PCR. The fragment was subcloned into the Mlu I site of the pEGFPC1, which was named as pU6-RZ596, and testified to be correct.
Healthy adult mice were spiled via portal vein after being anaesthetized and irrigated with a constant flux at about 9 mL/min. First Hanks solution was used without Ca2 + and Mg2 + , then 0.2 g/L collagenase solution for 8 min[11,12]. At last liver tissues were lacerated bluntly and digested vibrantly for 15 min in 37 °C bath water, and filtrated through nylon net and washed with PBS twice. Cells were resuspended in RPMI 1640 supplemented with 100 mL/L FBS and counted. Cell viability was 90%-92%. Cell count was adjusted to 2 × 106/mL and cells were cultured in a humidified 50 mL/L CO2 atmosphere at 37 °C. Cells were collected and grouped as following: empty control; cells transfected with pEGFPC1; cells transfected with pU6-RZ596. Transfection was performed according to the instructions of Effectene reagent. Cells were cultured for 48 h, the stably transfected cells were selected by culturing in medium containing G418 (600 μg/mL).
Total RNA was extracted from all above-mentioned groups by using TriZol reagent. After cDNA was synthesized, PCR reaction was performed, β-actin was used as control. The employed primers were as follows: Fas sense primer 5’-GCTGCAGACATGCTGTGGATC-3’ and anti-sense primer 5’-TCACAGCCAGGAGAATCGCAG-3’, β-actin sense primer 5’-GACGATGATATTGCCGCACT-3’ and anti-sense primer 5’-GATACCACGCTTGCTCTGAG-3’. Reaction conditions for PCR were: pre-denaturation for 3 min at 95 °C, 30 cycles of denaturation for 30 s at 95 °C, annealing for 45 s at 58 °C, extension for 45 s at 72 °C, and extension for 3 min at 72 °C. PCR products were run on 15 g/L agarose gel.
Three groups of cells were collected regularly and lysed in soluble buffer, then the proteins were measured as 0.4 μg/μL, and electrophoresed in 100 g/L SDS-polyacrylamide minigels. The first antibody was rabbit anti-mouse-Fas antibody (1:200). The second antibody was goat anti-rabbit-IgG (1:5000). At last the color was developed by ECL system.
Treated with JO2 (5 μg/mL) for 24 h under common growth condition, apoptosis of three-groups of cells were induced. Meanwhile the negative control without apoptosis and apoptosis cells treated with caspase-3 inhibitor (DEVD-fmk) were established. After being collected, cells were treated with cell lysis buffer sufficiently. The supernatant was retained (including protein needed), and then the procedure was performed according to the instructions of caspase-3 activity detection kit. The optical density (A) was measured at 405 nm.
Grouped and treated as above, cells were inoculated into 96-well plates at a concentration about 105/mL (100 μL/well). After incubation with 10 μL MTT (0.5 mg/mL) for 4 h at 37 °C, the formazan crystals were dissolved in 100 μL DMSO, then the A was measured at 570 nm.
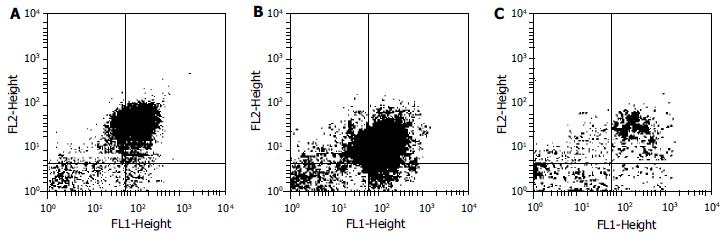
Three groups of cells treated with anti-Fas antibody JO2 and the empty control were conjugated with Annexin V-FITC and PI respectively. Cell apoptosis was analyzed by flow cytometry.
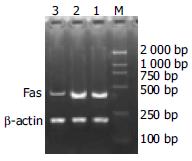
Fas and β-actin PCR amplification generated amplicons of 419 bp and 186 bp respectively. Through scanning the luminescence, the ratio of Fas/β-actin was analyzed by using gel image analysis system. The negative control was 1.1; mouse hepatocytes transfected with pEGFPC1 was 0.98; hepatocytes transfected with pU6-RZ596 was 0.43. It was clear that Fas mRNA expression in cells transfected with anti-Fas ribozyme was lower than that in control and cells transfected with empty vector (Figure 1).
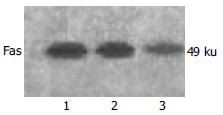
Western blotting displayed Fas protein expressed in cells transfected with pU6-RZ596 was much lower than that expressed in control and cells transfected with pEGFPC1, which was consistent with the results of RT-PCR (Figure 2).
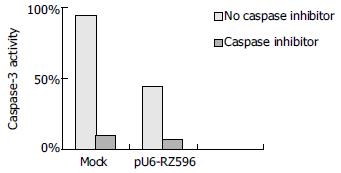
After 24 h of stimulation with JO2, caspase-3 activity of mouse hepatocytes was specifically inhibited by a caspase-3 inhibitor. Compared with negative control, caspase-3 activities of other groups transfected with pEGFPC1 and pU6-RZ596 were 95% and 45% respectively (Figure 3).
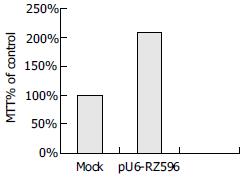
After induction of apoptosis of mouse hepatocytes through stimulation with JO2, cell viability was detected by MTT assay. Compared with negative control, cell viabilities of other groups transfected with pEGFPC1 and pU6-RZ596 were 98% and 208% respectively (Figure 4).
Cell apoptosis was induced as the method mentioned above. Apoptosis rates of cells in control, mock-transfected and pU6-RZ596-transfected groups were 86%, 87% and 35%, respectively (Figure 5).
Usually in clinic the hepatocytes of patients with acute or chronic liver failure show denaturation, necrosis and function failure. Existing pathogenic therapy and usual allopathy can not compensate functions of hepatocytes[12,13]. Now, the most efficient way is liver transplantation, but grafts survival is one of the important factors to affect whether liver transplantation is successful.
Primary mouse hepatocytes were isolated through two-step irrigation method[14], in which Fas expression was detected by RT-PCR and Western bloting. Treated with anti-Fas antibody JO2, apoptosis of hepatocytes was induced with decreased viability. So hammerhead ribozyme targeting directly at 596 GUA triplets of Fas mRNA was constructed to efficiently block out Fas gene and decrease apoptosis of hepatocytes. As an RNA molecule with catalysis function[15], ribozyme can bind and cleave target RNA to inhibit gene expression. Hammerhead ribozyme is widely used in gene therapy because of its many superiorities[16], including small molecule weight, easy to design and synthesis, etc. Anti-Fas ribozyme was transfected into mouse hepatocytes by using Effectene reagent, then the stable clone was selected to investigate the inhibition action of ribozyme on Fas-mediated apoptosis. Results displayed that expression of Fas mRNA and protein in cells transfected with anti-Fas ribozyme was remarkably lower than that in control and mock-transfected cells. After stimulation with apoptosis inducer JO2 (imitating the role of FasL in vivo) for 24 h, apoptosis rate of cells transfected with anti-Fas ribozyme was obviously lower than that of negative control and mock-transfected cells. Accordingly, caspase-3 activity of ribozyme-transfected cells was much lower than that of other groups, but cell viability was higher than the other two groups.
Apoptosis activates a series of proteases to induce programmed death of target cells through two ways including death receptor way and mitochondria way[17,18]. The final effect of the two ways is activity of caspase-3, that is to say caspase-3 is the common channel of two apoptosis ways[19]. Therefore, caspase-3 activity was increased in apoptosis and correlated with apoptosis rate positively. Anti-Fas ribozyme could not only decrease Fas expression but also weaken caspase-3 activity to reduce apoptosis induced by anti-Fas antibody. It is clear that hammerhead ribozyme targeting at 596 site of Fas RNA in this research can efficiently cleave Fas mRNA, which decreases not only the Fas expression but also its function. In existence of factors inducing apoptosis, ability of hepatocytes transfected with ribozyme against apoptosis was enhanced and cell viability was increased, which was coincident with study results of Dagmer about primary mouse islet β cells and insulinoma cells[20,21].
In short, anti-Fas ribozyme not only prevents Fas expression in mouse hepatocytes but also inhibits Fas-mediated apoptosis to improve cell survival after being transfected into hepatocytes, which afford strong assurance for successful liver transplantation. Further research should be focused on clinical application of anti-Fas ribozyme and selection of more efficient, highly specific and low toxic vectors[22-24].
Edited by Zhu LH and Chen WW Proofread by Xu FM
| 1. | Sato Y, Yamamoto S, Takeishi T, Nakatsuka H, Kokai H, Hatakeyama K. New hepatic vein reconstruction by double expansion of outflow capacity of left-sided liver graft in living-donor liver transplantation. Transplantation. 2003;76:882-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Akamatsu N, Sugawara Y, Kaneko J, Sano K, Imamura H, Kokudo N, Makuuchi M. Effects of middle hepatic vein reconstruction on right liver graft regeneration. Transplantation. 2003;76:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Sorom AJ, Nyberg SL, Gores GJ. Keratin, fas, and cryptogenic liver failure. Liver Transpl. 2002;8:1195-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Rivero M, Crespo J, Mayorga M, Fábrega E, Casafont F, Pons-Romero F. Involvement of the Fas system in liver allograft rejection. Am J Gastroenterol. 2002;97:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Uchiyama H, Kishihara K, Minagawa R, Hashimoto K, Sugimachi K, Nomoto K. Crucial Fas-Fas ligand interaction in spontaneous acceptance of hepatic allografts in mice. Immunology. 2002;105:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Wang H, Chen XP, Qiu FZ. Overcoming multi-drug resistance by anti-MDR1 ribozyme. World J Gastroenterol. 2003;9:1444-1449. [PubMed] |
| 7. | Dunham CM, Murray JB, Scott WG. A helical twist-induced conformational switch activates cleavage in the hammerhead ribozyme. J Mol Biol. 2003;332:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Nikcevic G, Kovacevic-Grujicic N, Stevanovic M. Improved transfection efficiency of cultured human cells. Cell Biol Int. 2003;27:735-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Persson T, Hartmann RK, Eckstein F. Selection of hammerhead ribozyme variants with low Mg2 + requirement: importance of stem-loop II. Chembiochem. 2002;3:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Murray JB, Dunham CM, Scott WG. A pH-dependent conformational change, rather than the chemical step, appears to be rate-limiting in the hammerhead ribozyme cleavage reaction. J Mol Biol. 2002;315:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Azuma H, Hirose T, Fujii H, Oe S, Yasuchika K, Fujikawa T, Yamaoka Y. Enrichment of hepatic progenitor cells from adult mouse liver. Hepatology. 2003;37:1385-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci. 2003;116:1775-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 272] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Wagner M, Kaufmann P, Fickert P, Trauner M, Lackner C, Stauber RE. Successful conservative management of acute hepatic failure following exertional heatstroke. Eur J Gastroenterol Hepatol. 2003;15:1135-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Crossin JD, Muradali D, Wilson SR. US of liver transplants: normal and abnormal. Radiographics. 2003;23:1093-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Heneghan MA, Zolfino T, Muiesan P, Portmann BC, Rela M, Heaton ND, O'grady JG. An evaluation of long-term outcomes after liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2003;9:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Musallam L, Ethier C, Haddad PS, Denizeau F, Bilodeau M. Resistance to Fas-induced apoptosis in hepatocytes: role of GSH depletion by cell isolation and culture. Am J Physiol Gastrointest Liver Physiol. 2002;283:G709-G718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Steitz TA, Moore PB. RNA, the first macromolecular catalyst: the ribosome is a ribozyme. Trends Biochem Sci. 2003;28:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Khan AU, Lal SK. Ribozymes: a modern tool in medicine. J Biomed Sci. 2003;10:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Shi G, Wu Y, Zhang J, Wu J. Death decoy receptor TR6/DcR3 inhibits T cell chemotaxis in vitro and in vivo. J Immunol. 2003;171:3407-3414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Curtin JF, Cotter TG. Live and let die: regulatory mechanisms in Fas-mediated apoptosis. Cell Signal. 2003;15:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Ientile R, Campisi A, Raciti G, Caccamo D, Currò M, Cannavò G, Li Volti G, Macaione S, Vanella A. Cystamine inhibits transglutaminase and caspase-3 cleavage in glutamate-exposed astroglial cells. J Neurosci Res. 2003;74:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Klein D, Denis M, Ricordi C, Pastori RL. Assessment of ribozyme cleavage efficiency using reverse transcriptase real-time PCR. Mol Biotechnol. 2000;14:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Klein D, Ricordi C, Pugliese A, Pastori RL. Inhibition of Fas-mediated apoptosis in mouse insulinoma betaTC-3 cells via an anti-Fas ribozyme. Hum Gene Ther. 2000;11:1033-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Mergia A, Heinkelein M. Foamy virus vectors. Curr Top Microbiol Immunol. 2003;277:131-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |