Published online Sep 1, 2004. doi: 10.3748/wjg.v10.i17.2563
Revised: February 19, 2004
Accepted: February 24, 2004
Published online: September 1, 2004
AIM: To investigate the feasibility for antisense imaging of the colon cancer with liposome-entrapped 99 m-technetium labeled antisense oligonucleotides as tracers.
METHODS: Fifteen mer single-stranded aminolinked phosphorothioate antisense oligonucleotides of c-myc mRNA were labeled with 99mTc-pertechnetate, then purified and finally entrapped with liposomes to form the labeling compounds, liposome-entrapped 99mTc-labeled antisense oligonucleotides. The LS-174-T cells (colon of adenocarcinoma cell line) were incubated with the labeling compounds to test the uptake rates of LS-174-T cells. Later on, a model of 30 tumor bearing nude mice was constructed by inoculating with 5 × 106 of LS-174-T cells at right flank of each nude mouse. About 10 d later, the model were adminstered by intravenous injection of the liposome-entrapped 99mTc-labeled antisense oligonucleotides. Then some of the tumour bearing nude mice were sacrificed at 0.5, 1, 2, and 4 h after intravenous injection, and proper quantity of liver, spleen, tumor, etc. was obtained. The tissues were counted in a gamma counter, and after correction for decay and background activity, expressed as a percentage of the injected dose. The others whose anterior and posterior whole-body scans were obtained at 1, 1.5, 2, 4, 6 and 24 h with a dual-head bodyscan camera equipped with parallel-hole low-energy collimaters. The ratios of radioactive counts in tumor to that in contralateral equivalent region of abdomen were calculated.
RESULTS: The uptake rates of LS-174-T cells for liposome-entrapped 99mTc-labeled antisense oligonucleotides increased as time prolonged and reach the peak (17.77% ± 2.41%) at 7 h. The biodistributions showed that the rdioactivity in the tumor (13.46% ± 0.20%) of injected dose was the highest at 2 h of intravenous injection of liposome-entrapped 99mTc-labeled antisense oligonucleotides, and then decreased sharply to 4.58% ± 0.45% at 4 h. The tumor was shown clearly in the whole-body scan at 2 h of intravenous injection. The ratios, radioactive counts in tumor to that in contralateral equivalent region of abdomen (1.7332 ± 0.2537), was the highest one at 2 h after intravenous injection of liposome-entrapped 99mTc-labeled antisense oligonucleotides.
CONCLUSION: The liposome-entrapped 99mTc-labeled antisense oligonucleotides deserve being developed into radiopharmaceutics for the colon cancer imaging.
-
Citation: Zheng JG, Tan TZ. Antisense imaging of colon cancer-bearing nude mice with liposome-entrapped 99m-technetium-labeled antisense oligonucleotides of
c-myc mRNA. World J Gastroenterol 2004; 10(17): 2563-2566 - URL: https://www.wjgnet.com/1007-9327/full/v10/i17/2563.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i17.2563
Antisense imaging was referred to that antisense oligonucleotides of a gene were labeled with radionuclide, then administered to a organism to show its focus, especially the tumor[1]. Colon cancer is a malignant tumor that seriously threatens human health. Its main oncogene, c-myc, whose overexpression can reach 30 times, is a target gene for antisense imaging[2]. The oligonucleotides that are complementary to c-myc mRNA can prohibit many kinds of cancer cells from growing[3]. Many nuclear medicine researchers are interested in this oncogene[4,5]. At present, the antisense oligonucleotides of c-myc mRNA have been successfully labeled with 99mTc [6,7]. However, to the author’s knowledge, their application in experimental researches on antisense imaging has not been reported as yet. Is the uptake rate of tumor tissue too small and the background too high to indicate the tumor How can the uptake rates of tumor cells be increased Do these limit the application of 99mTc-labeled antisense oligonucleotides In order to explore the feasibility for antisense imaging of the colon carcinoma, develop a new radiolabeled-gene-pharmaceutics, and promote the progress in the molecular nuclear medicine, the primary experimental studies, antisense imaging, on liposome-entrapped 99mTc-labeled antisense phosphorothioate oligonucleotides as a tracer were carried out.
Fifteen mer, single-stranded phosphorothioate oligonucleotides, aminolinked, antisense oligonucleotides targeted at the translation initiation codon of c-myc mRNA were purchased from Gibco-BRL, US. Their base sequences were 5’-NH2-FACGTTGAGGGGCAT-3’ (F stood for phosphorothioate A).The molecular mass of the chain was about 300 u. These oligonucleotides were used directly without further purification, and generally handled under sterile conditions. All solutions were sterilized by terminal filtration through a 0.22 μm filter. All pipettes and tubes were autoclaved prior to use. The oligonucleotides were dissolved at a concentration of 4 mg/mL in sterile water and stored at -20 °C.
The hydrazino nicotinamide derivatives were synthesized elsewhere. 99 m-pertechnetate was obtained from a 99mo-99mTc radionuclide generator made by the Chinese Atomic Energy Institute. Tricine, Sncl2·2H2O and Dimethyl sulfoxide were supplied by Sigma Company, US, lipofectamin reagent by Gibco-BRL, US, EDTA by Boehringer Mannheim Company, Germany, and Sep-Pak (C18) reverse column by Waters Company, US.
The oligonucleotides were bound to hydrazino nicotinamide derivatives and then labeled with 99mTc following the methods described by Hnatowich et al[8]. The 99mTc-labeled oligonucleotides were entrapped with liposome according to the manufacturer’s protocols.
The LS-174-T cells were grown by adherent culture in media (RPMI-1640, Gibco-BRL, US), supplemented with 100 mL/L fetal bovine serum at 37 °C, 50 mL/L CO2. Thirty-six culture plates with diameter of 33 mm each was inoculated with about 1 × 105 LS-174-T cells and cultured at 37 °C for 48 h. After the cells were grown to about 50% confluence in regular culture media, they were transfected using lipofectamin with 2 μg of freshly prepared liposome-entrapped 99mTc-labeled antisense oligonucleotides with radioactivity of about 29.60 MBq according to the manufacturer’s protocols. The cellular uptake rates were determined at 1, 2, 5 and 7 h, and the testing steps were as follows: The cells were detached by 2.5 g/L trypsin to form suspension, then washed three times with the media by centrifugation (2500 r/min, 10 min). The supernatant was collected into a 50 mL volumetric cylinder and the precipitation remained in the centrifugation tube. Then the radioactive counts in the precipitation and supernatant were counted in an automatic gamma well counter after correction for decay and background activity separately, and the cellular uptake rates were expressed as a percentage of the total counts. The uptake rate = radioactive counts in precipitation/the total counts in precipitation and supernatant × 100%.
At first, the tumor model was constructed. Large-scale of LS-174-T cells collected by digestion, centrifugation and washing, were diluted with culture medium without serum and antibiotic to the concerntration of 5 × 106 cells per 0.2 mL. Thirty male nude mice, aged about 2 mo, were purchased from Experimental Animal Center of Sichuan University. The mice were maintained in a specific pathogen-free environment and cared in accordance with the institutional guidelines. Each of them was inoculated with 5106 cells at right flank. The tumor was allowed to grow for 10 d until diameter reached about 1 cm. Thus the tumor model was constructed successfully.
Biodistribution of liposome-entrapped 99mTc-labeled antisense oligonucleotides was determined in the 20 tumor-bearing nude mice. Each mouse received 0.3 mL of saline containing 3 to 5 μg (0.259 MBq) of liposome-entrapped 99mTc-labeled antisense oligonucleotides by tail vein administration. Meanwhile, the same injected dose of liposome-entrapped 99mTc-labeled antisense oligonucleotides was stored in a tube as standard, and double assays were carried out. Five mice were used at each time point of 0.5, 1, 2 and 4 h. After being bled from eye, they were sacrificed by cervical dislocation, and proper quantity of liver, spleen, kidney, lung, heart, bone, muscle, stomach, intestines, brain and tumor obtained. The tissues were washed cleanly with cool physiological saline and weighted by electronic balance (Denver, US). The tissues were counted against appropriate standards of known dilution in an automatic gamma well counter, and after correction for decay and background activity, expressed as a percentage of the injected dose.
The liposome-entrapped 99mTc-labeled antisense oligonucleotides containing oligonucleotides from 30 to 90 μg at the concentration of 37 MBq/mL were freshly prepared. The 6 tumor-bearing nude mice were administered with 0.3 mL of the above products through tail vein. When these mice anesthetized with pentobarbital, anterior and posterior whole-body scans were obtained at 1, 1.5, 2, 4, 6 and 24 h with a dual-head bodyscan camera (Elcint Apex Helix, Israel) equipped with parallel-hole low-energy collimaters. The ratios of radioactive counts in tumor to that in the contralateral equivalent region of abdomen were calculated.
The most important thing for antisense imaging is how much oligonucleotides are able to enter cells. High uptake rate is the key to success. The celluar uptake rates are as follows: 7.21% ± 1.23% at 1 h, 15.19% ± 2.81% at 2 h, 16.13% ± 2.54% at 5 h, and 17.77% ± 2.41% at 7 h. Within 7 h, the cellular uptake rate increased as the time prolonged, and reached the peak at 7 h. It was significantly higher than that at 1 and 2 h. However, the uptake rate at 7 h was not significantly higher than that at 5 h.
The biodistribution of liposome-entrapped 99mTc-labeled antisense oligonucleotides are shown in Table 1. The endothelial system played a main role in biodistribution, and accumulated the greater part of the injected dose. The radioactive counts in the tumor tissue increased within 2 h and gradually reached the peak at 2 h, then dropped down sharply.
| Tissue | 0.5 h | 1 h | 2 h | 4 h |
| Liver | 8.78 ± 0.63 | 9.16 ± 1.14 | 7.92 ± 0.38c | 8.97 ± 0.12c |
| Spleen | 6.78 ± 0.37c | 8.86 ± 0.60 | 7.37 ± 0.64c | 8.02 ± 0.23c |
| Kidney | 4.89 ± 0.67a | 2.90 ± 1.19a | 3.07 ± 0.18ac | 0.47 ± 0.02ac |
| Lung | 10.23 ± 1.02 | 13.37 ± 0.84 | 12.21 ± 0.42a | 10.20 ± 0.50c |
| Heart | 7.18 ± 0.13 | 8.78 ± 1.01 | 7.25 ± 0.18 | 5.28 ± 0.45ac |
| Blood | 5.51 ± 0.24a | 4.55 ± 0.15 | 2.81 ± 0.11ac | 2.61 ± 0.06ac |
| Bone | 1.84 ± 0.64a | 3.14 ± 1.04a | 1.43 ± 0.24ac | 1.02 ± 0.32ac |
| Muscle | 2.27 ± 0.37a | 1.36 ± 0.60ac | 1.69 ± 0.91ac | 2.85 ± 0.26a |
| Stomach | 12.45 ± 0.62a | 13.77 ± 0.38 | 10.63 ± 0.35 | 6.70 ± 0.44ac |
| Intestines | 4.54 ± 0.42a | 6.68 ± 4.14 | 7.76 ± 0.34c | 8.44 ± 0.63c |
| Brain | 2.24 ± 0.42a | 2.16 ± 2.10ac | 0.68 ± 0.06ac | 0.52 ± 0.06ac |
| Tumor | 6.12 ± 0.31a | 8.09 ± 0.86 | 13.46 ± 0.20a | 4.58 ± 0.45a |
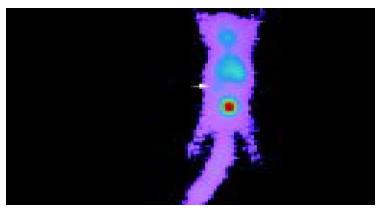
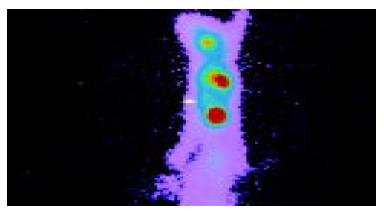
Anterior imaging at 1 h after intravenous injection of liposome-entrapped 99mTc-labeled antisense oligonucleotides showed that little accumulation of radioactivity might be seen in the right middle flank which was the place of tumor. Anterior imaging demonstrated a little accumulation of radioactivity in tumor site at 1.5 h (Figure 1), and a circular abnormal accumulation focus of radioactivity in the location of tumor at 2 h (Figure 2), but no accumulation of radioactivity in the location of tumor at 24 h.
The ratio was the highest one (1.7332 ± 0.2537) at 2 h after intravenous injection of liposome-entrapped 99mTc-labeled antisense oligonucleotides. It was significantly higher than that at 4 and 6 h (P < 0.05). Although it was higher than that at 1.0 and 1.5 h, there was no statistical difference. These ratios are shown in Table 2.
The possible advantages of delivering oligonucleotides into cells by liposomes are not only to increase the uptake rates of dividing and mitostatic cells, but also protect the oligonucleotides against degeneration by nucleases. It has been confirmed that the amount of oligonucleotides used can be decreased greatly as liposomes are adopted as vectors in vitro[9-13].
The cellular uptake rates of the liposome-entrapped 99mTc-labeled antisense oligonucleotides are the key parameter to antisense imaging. The maxium cellular uptake rate of the LS-174-T (17.77% ± 2.41%) was plenty enough for the experimental or clinical use, and could be applied for the following experiments: biodistribution and antisense imaging.
The biodistribution assay showed that the radioactive counts per gram of the tumor tissue was in the middle level at 0.5 h after injection, increased as time prolonged within 2 h, and reached the peak at 2 h. The proportion of radioactive counts per gram of the tumor tissue to the total was 13.46% ± 0.20%, which was significantly higher than that of the liver, spleen, kidney, blood, bone, muscle, intestines and brain. The ratio of radioactive counts in the tumor to that in the blood was 4.79, and to that in the muscle was 7.96. It was thus evident that enough liposome-entrapped 99mTc-labeled antisense oligonucleotides were accumulated in the tumor tissue. The tumor could be observed clearly so long as radioactivity in the tissues around it was comparatively low.
Delong et al[14]. studied the biodistribution of 99mTc-labeled phosphorothioate oligonucleotides without using liposomes as carriers. Their investigation demonstrated that radioactivity in the tumor tissue was only 2% to 3%. However, in our study, liposomes were used as vectors, and 4.58% to 13.46% of the total radioactivity could be accumulated in the tumor. Radioactivity was 2.29 to 4.49 times higher than that reported by Delong et al[14]. Thus, the uptake rates of the tumor tissue can be increasee greatly by using liposomes as carriers. Accordingly, liposome is an effective vector in antisense imaging.
The whole body scan in the tumor bearing nude mice showed that the tumor was observed clearly at 2 h after intravenous injection of the liposome-entrapped 99mTc-labeled antisense oligonucleotides. It was evident that the imaging of the liver above the tumor and the bladder below the tumor was more clear than that of the tumor. They could influence the imaging of the tumor. However, the interference of the bladder can easily be decreased by diuretic or drinking a certain quantity of water, which is a routine clinical method of whole body bone scan. But the influence of the liver needs further investigation.
Although the imaging of the liver will influence the quality of the antisense imaging, our primary success on the tumor-bearing nude mice with 99mTc-labeled antisense oligonuceotides may contribute to the molecular nuclear medicine progress.
In conclusion, liposome-entrapped 99mTc-labeled antisense oligonucleotides with high cellular uptake rates can be accumulated by tumor in the tumor bearing-nude mice, and be able to show the colon carcinoma accurately, was worthy of being developed into a new radiopharmaceutics for diagnosis[15]. This will provide a new strategy for the early diagnosis of the colon carcinoma.
Edited by Kumar M Proofread by Xu FM
| 1. | Gauchez AS, Du Moulinet D'Hardemare A, Lunardi J, Vuillez JP, Fagret D. Potential use of radiolabeled antisense oligonucleotides in oncology. Anticancer Res. 1999;19:4989-4997. [PubMed] |
| 2. | Zheng J, Tan T. The application of radionuclide antisense therapy for malignant tumours. Nucl Med Commun. 2001;22:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Kang Y, Cortina R, Perry RR. Role of c-myc in tamoxifen-induced apoptosis estrogen-independent breast cancer cells. J Natl Cancer Inst. 1996;88:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Dewanjee MK, Ghafouripour AK, Kapadvanjwala M, Dewanjee S, Serafini AN, Lopez DM, Sfakianakis GN. Noninvasive imaging of c-myc oncogene messenger RNA with indium-111-antisense probes in a mammary tumor-bearing mouse model. J Nucl Med. 1994;35:1054-1063. [PubMed] |
| 5. | Hjelstuen OK, Tønnesen HH, Bremer PO, Verbruggen AM. 3'-99mTc-labeling and biodistribution of a CAPL antisense oligodeoxynucleotide. Nucl Med Biol. 1998;25:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Zhang YM, Rusckowski M, Liu N, Liu C, Hnatowich DJ. Cationic liposomes enhance cellular/nuclear localization of 99mTc-antisense oligonucleotides in target tumor cells. Cancer Biother Radiopharm. 2001;16:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Liu G, Zhang S, He J, Liu N, Gupta S, Rusckowski M, Hnatowich DJ. The influence of chain length and base sequence on the pharmacokinetic behavior of 99mTc-morpholinos in mice. Q J Nucl Med. 2002;46:233-243. [PubMed] |
| 8. | Hnatowich DJ, Mardirossian G, Fogarasi M, Sano T, Smith CL, Cantor CR, Rusckowski M, Winnard P. Comparative properties of a technetium-99m-labeled single-stranded natural DNA and a phosphorothioate derivative in vitro and in mice. J Pharmacol Exp Ther. 1996;276:326-334. [PubMed] |
| 9. | Ma DD, Wei AQ. Enhanced delivery of synthetic oligonucleotides to human leukaemic cells by liposomes and immunoliposomes. Leuk Res. 1996;20:925-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Ropert C, Lavignon M, Dubernet C, Couvreur P, Malvy C. Oligonucleotides encapsulated in pHsensitive liposomes are efficient toward Friend retrovirus. Biochem Biophys Res Commun. 1992;183:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Zelphati O, Zon G, Leserman L. Inhibition of HIV-1 replication in cultured cells with antisense oligonucleotides encapsulated in immunoliposomes. Antisense Res Dev. 1993;3:323-338. [PubMed] |
| 12. | Kanamaru T, Takagi T, Takakura Y, Hashida M. Biological effects and cellular uptake of c-myc antisense oligonucleotides and their cationic liposome complexes. J Drug Target. 1998;5:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Moreira JN, Hansen CB, Gaspar R, Allen TM. A growth factor antagonist as a targeting agent for sterically stabilized liposomes in human small cell lung cancer. Biochim Biophys Acta. 2001;1514:303-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | DeLong RK, Nolting A, Fisher M, Chen Q, Wickstrom E, Kligshteyn M, Demirdji S, Caruthers M, Juliano RL. Comparative pharmacokinetics, tissue distribution, and tumor accumulation of phosphorothioate, phosphorodithioate, and methylphosphonate oligonucleotides in nude mice. Antisense Nucleic Acid Drug Dev. 1997;7:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Zheng J, Tan T, Zhang C, Li Y, Liang Z, Tu J. [Preparation of liposome-mediated 99m-technetium-labeled antisense oligonucleotides of c-myc mRNA]. Shengwu Yixue Gongchengxue Zazhi. 2003;20:704-707. [PubMed] |