Published online Sep 1, 2004. doi: 10.3748/wjg.v10.i17.2550
Revised: January 4, 2004
Accepted: January 15, 2004
Published online: September 1, 2004
AIM: To observe the plasticity of whether dermis-derived multipotent cells to differentiate into insulin-producing pancreatic cells in vitro.
METHODS: A clonal population of dermis-derived multipotent stem cells (DMCs) from newborn rat with the capacity to produce osteocytes, chondrocytes, adipocytes and neurons was used. The gene expression of cultured DMCs was assessed by DNA microarray using rat RGU34A gene expression probe arrays. DMCs were further cultured in the presence of insulin complex components (Insulin-transferrin-selenium, ITS) to observe whether DMCs could be induced into insulin-producing pancreatic cells in vitro.
RESULTS: DNA microarray analysis showed that cultured DMCs simultaneously expressed several genes associated with pancreatic cell, neural cell, epithelial cell and hepatocyte, widening its transcriptomic repertoire. When cultured in the specific induction medium containing ITS for pancreatic cells, DMCs differentiated into epithelioid cells that were positive for insulin detected by immunohistochemistry.
CONCLUSION: Our data indicate that dermal multipotent cells may serve as a source of stem/progenitor cells for insulin-producing pancreatic cells.
-
Citation: Shi CM, Cheng TM. Differentiation of dermis-derived multipotent cells into insulin-producing pancreatic cells
in vitro . World J Gastroenterol 2004; 10(17): 2550-2552 - URL: https://www.wjgnet.com/1007-9327/full/v10/i17/2550.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i17.2550
Organ-specific stem cells possess plasticity that can permit differentiation along new lineages and have significant implications for future therapies of diabetes. The production of endocrine pancreas and insulin-secreting beta cells from adult nonpancreatic stem cells and hepatic oval stem cell has been demonstrated[1,2]. Zulewski et al[2] also showed that pancreatic islets contained a heretofore unrecognized distinct population of cells that expressed the neural stem cell-specific marker nestin. The nestin-positive islet-derived progenitor (NIP) cells are a distinct population of cells that reside within pancreatic islets and may participate in the neogenesis of islet endocrine cells[2]. According to this result, liver stem cells have been proved to differentiate into pancreatic islet-like cells[3]. Dermis is a highly accessible tissue source for adult stem cells. Nestin-positive skin-derived stem cells have been isolated from dermis by Toma et al[4]. In our previous study, we isolated a clonal population of dermal multipotent cells by their adherence to tissue culture plastic (termed as plastic adherent DMCs) from newborn rat dermis. These cells showed the differentiation capacity to produce nestin-positive cells[5]. In this study, we aimed to investigate whether plastic adherent DMCs had the differentiation capacity to produce insulin-producing pancreatic cells in vitro.
A clonal population of dermis-derived multipotent stem cells (DMCs) was used in the experiments. The clonal population of DMCs were isolated and identified from primary dermal cells of male newborn Wistar rat as previously described[5]. All tissue culture reagents, including Iscove’s Modified Dulbecco’s Medium (IMDM), ITS (insulin-transferrin selenium), epidermal growth factor, basic fibroblast growth factor, were purchased from Sigma (St. Louis, CA, USA). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA). The rat RGU34A gene expression probe arrays were purchased from Affymetrix (Santa Clara, CA). Mouse anti-insulin monoclonal antibody was purchased from Sigma (St. Louis, CA, USA). Horseradish peroxidase (HRP) -labeled goat anti-mouse IgG antibody was purchased from Boster (Wuhan, China).
Cell culture The DMCs were cultured in IMDM medium containing 10 μL/L fetal bovine serum and 100 U/mL penicillin and 100 μg/mL streptomycin. Cultures were maintained at 37 °C in a humidified atmosphere containing 50 mL/L CO2. For differentiation induction, DMCs were transferred into specific inducing medium containing 10 μg/mL of keratocyte growth factor (KGF), 20 ng/mL of epidermal growth factor (EGF), 10 mmol/L of nicotinamide, and 1 mg/mL of ITS. The differentiated cells were examined for the insulin expression by immunohistochemistry.
DNA Microarray analysis Total RNA from cultured DMCs was isolated using QIAGEN’s RNeasy total RNA isolation kit (Rneasy, Qiagen) following the manufacture’s instructions, and quantified. Transcript profiling was conducted by means of rat RGU34A gene expression probe arrays, containing 8799 probe sets, interrogating primarily annotated genes. The experiment was conducted according to the recommendations of the manufacturer (Affymetrix GeneChip expression analysis manual). The resulting “data-file” was processed further using the microarray analysis suite 5 software package (Affymetrix). According to the statistical expression analysis algorithm, the presence of a gene within a given sample was determined at a detection P value of < 0.05 and was graded as absent (A), marginal (M), or present/positive (P).
Immunocytochemistry Immunocytochemistry study with peroxidase-labeled streptavidin biotin method was performed to detect the expression of insulin in differentiated cells. Before detection, the cells were washed three times for 20 min with phosphate-buffered saline (PBS, pH = 7.2) to exclude the pollution of exogenous insulin in the culture medium. After washing, the cells were blocked for 30 min with 100 mL/L normal goat serum in PBS and were incubated with mouse anti-insulin antibody (1:100) at 4 °C for 24 h. Incubation at room temperature with anti-mouse secondary antibody and avidin-biotinylated peroxidase complexes was performed for 2 h. The specimens were washed for 15 min with 0.01 mol/L PBS between all steps. The reaction product was developed with 0.5 g/L 3’3-diaminobenzine tetrahydrochloride (DAB). After staining, the result was observed microscopically. PBS was used as a substitute for primary antibody as negative control.
Most adherent DMCs were of spindle-shaped cells (Figure 1). Phenotype analysis showed that DMCs were negative for some lineage-specific surface markers, including pan-cytokeratin, cytokeratin19, factor VIII, CD31, CD45, CD34, α-smooth muscle actin (α-SMA), desmin, collagen II and nestin, but were positive for CD59, CD90, CD44, vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). The doubling time of cultured DMCs was about 40 h. Following induction, DMCs had the capacity to differentiate into cells with phenotypic characteristics of osteocytes (alkaline phosphatase activity and alizarin red staining), adipocytes (oil red staining), chondrocytes (collagen II and Alcian blue staining) and neurons (nestin and NF-200 staining) in specific induction media.
The gene expression of DMCs was assessed by DNA microarray analysis and the harbinger of a potential cell type was viewed by simultaneous expression of a panel of lineage-related genes. The result revealed that the cultured DMCs simultaneously expressed genes associated with pancreatic cells (Table 1). DMCs also simultaneously expressed transcripts for epithelial cell, neural cell, and hepatocyte as well (Table 1). These transcripts were developmentally related with pancreatic tissue and further widened the transcriptomic repertoire of DMCs. To confirm the data by DNA microarray, several genes were further tested by RT-PCR and the result was consistent with the result from microarray (data not shown).
| Cell lineage | Representative examples of associated genes |
| A: Pancreatic cell | Pancreatic eukaryotic initiation factor 2 alpha-subunit kinase (PEK), pancreatic secretory trypsin inhibitor-like protein (PSTI), pancreatic islet cDNA Rattus norvegicus cDNA similar to rapamycin-binding protein FKBp-13, insulin II gene, IRS-1 mRNA for insulin-receptor,mRNA for glucose-dependent insulinotropic polypeptide |
| B: Neural cell | Neural visinin-like Ca2 + -binding protein type 3, neuron-specific enolase, neuronal nitric oxide synthase, GABA receptor-associated proteins, NCAM, brain-derived neurotrophic factor (BDNF), syntaxin 7, glial fibrillary acidic protein (GFAP), adrenomedullin, myelin protein SR13 (growth-arrest-specific Gas-3 homolog), neurotrimin; secretogranin II, synapse-associated protein 102, latexin, kexin-like protease PC7A, N-methyl-D-aspartate (NMDA) receptor |
| C: Epithelial cell | Epithelial membrane protein 1, epithelial cell transmembrane protein antigen precursor, epidermal growth factor precursor, heparin-binding EGF-like growth factor , mucin, parathymosin and thy mosin 4 and 10 |
| D: Hepatocyte | Liver glycogen phosphorylase enzyme, liver IL-6 receptor ligand binding chain, liver specifc tran scription factor LF-B, liver nuclear protein P47, liver aL-Fucosidase, hepatocyte nuclear factor 3a (HNF-3beta) |
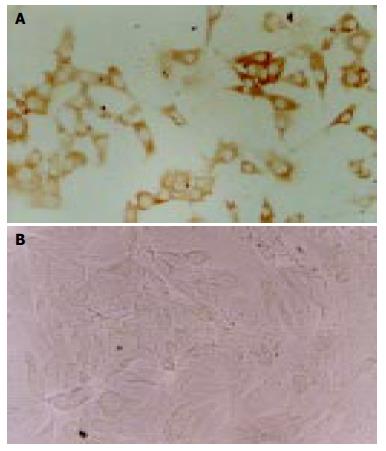
DMCs were further tested for their capacity to produce pancreatic cells in the inducing medium containing ITS. The morphology of DMCs were changed into epithelial cells-like cells when cultured in medium containing ITS for 2 wk and immunohistochemistry showed that a proportion of cells (less than 10%) were positive for insulin when cultured in medium containing ITS for 4 wk (Figure 2). The negative control cells showed no positive staining for insulin. This result further indicated that DMCs could undergo differentiation to form insulin-producing cells.
Replacement of the insulin-producing pancreatic islet β cells represents the ultimate treatment for type I diabetes. Recent advances in islet transplantation underscore the urgent need for developing alternatives to human tissue donors, which are scarce. The generation of insulin-producing cells from adult stem cells is one possible approach[6-9]. Recent studies have suggested that there are closely developmental relationship between the nestin positive progenitors and the pancreatic cells[10-13]. In addition to liver stem cells and neural stem cells, multipotent stem cells from dermis have been proved to have the capacity to produce nestin positive cells[4,5]. In this study, we further reported that dermis-derived multipotent stem cells also showed a remarkable flexibility to differentiate into insulin-producing cells, when the appropriate stimuli were given. Since dermal cells are relatively easy to access and they also can be used for autologous use that may represent a safety advantage, dermis-derived multipotent cells may serve as a source of stem/progenitor cells for β cells.
Nevertheless, the efficiency of adult stem cells to differentiate into insulin-producing cells in vitro is low at present and expansion with large scale is needed for application. Genetic manipulation in tissue culture may be a choice and it is possible that new insights into endocrine pancreas development will lead to the manipulation of progenitor cell fate towards the β cell phenotype of insulin production, storage and regulated secretion[14,15]. If successful, these approaches could lead to widespread cell replacement therapy for type I diabetes.
Edited by Kumar M Proofread by Xu FM
| 1. | Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H, Taniguchi H. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J Cell Biol. 2002;156:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 553] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 3. | Meivar-Levy I, Ferber S. New organs from our own tissues: liver-to-pancreas transdifferentiation. Trends Endocrinol Metab. 2003;14:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1125] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 5. | Shi C, Cheng T. Effects of acute wound environment on neonatal rat dermal multipotent cells. Cells Tissues Organs. 2003;175:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Street CN, Rajotte RV, Korbutt GS. Stem cells: a promising source of pancreatic islets for transplantation in type 1 diabetes. Curr Top Dev Biol. 2003;58:111-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Scharfmann R. Alternative sources of beta cells for cell therapy of diabetes. Eur J Clin Invest. 2003;33:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Soria B, Skoudy A, Martín F. From stem cells to beta cells: new strategies in cell therapy of diabetes mellitus. Diabetologia. 2001;44:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Pattou F, Kerr-Conte J, Gmyr V, Vandewalle B, Vantyghem MC, Lecomte-Houcke M, Proye C, Lefebvre J. [Human pancreatic stem cell and diabetes cell therapy]. Bull Acad Natl Med. 2000;184:1887-1899; discussion 1887-199;. [PubMed] |
| 10. | Delacour A, Nepote V, Trumpp A, Herrera PL. Nestin expression in pancreatic exocrine cell lineages. Mech Dev. 2004;121:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Esni F, Stoffers DA, Takeuchi T, Leach SD. Origin of exocrine pancreatic cells from nestin-positive precursors in developing mouse pancreas. Mech Dev. 2004;121:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Humphrey RK, Bucay N, Beattie GM, Lopez A, Messam CA, Cirulli V, Hayek A. Characterization and isolation of promoter-defined nestin-positive cells from the human fetal pancreas. Diabetes. 2003;52:2519-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Lardon J, Rooman I, Bouwens L. Nestin expression in pancreatic stellate cells and angiogenic endothelial cells. Histochem Cell Biol. 2002;117:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Suzuki T, Kadoya Y, Sato Y, Handa K, Takahashi T, Kakita A, Yamashina S. The expression of pancreatic endocrine markers in centroacinar cells of the normal and regenerating rat pancreas: their possible transformation to endocrine cells. Arch Histol Cytol. 2003;66:347-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 546] [Article Influence: 21.8] [Reference Citation Analysis (0)] |