Published online Aug 15, 2004. doi: 10.3748/wjg.v10.i16.2419
Revised: December 22, 2004
Accepted: January 15, 2004
Published online: August 15, 2004
AIM: To evaluate the effect of parenteral and enteral nutrition combined with octreotide on pancreatic exocrine secretion of the patients with pancreatic fistula.
METHODS: Pancreatic juice, drained directly from the pancreatic fistula, was collected, and the volume, protein, amylase, HCO3-, K+, Na+ and Cl- were determined on d 1, 4 and 7 before and after 7-d treatment with octreotide, respectively.
RESULTS: No differences in exocrine pancreatic secretion were observed during the enteral and parenteral nutrition period (t = 2.03, P > 0.05); there were significant decreases in pancreatic juice secretion volume, protein, amylase, HCO3-, K+, Na+ and Cl- after parenteral and enteral nutrition combined with octreotide compared with octreotide pretreatment (t = 4.14, P < 0.05).
CONCLUSION: There is no stimulatory effect on the pancreatic secretion by intrajejunal nutrition and parenteral nutrition. Octreotide is effective on the reduction of pancreatic fistula output.
- Citation: Qin HL, Su ZD, Zou Y, Fan YB. Effect of parenteral and enteral nutrition combined with octreotide on pancreatic exocrine secretion of patients with pancreatic fistula. World J Gastroenterol 2004; 10(16): 2419-2422
- URL: https://www.wjgnet.com/1007-9327/full/v10/i16/2419.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i16.2419
Since the 1960 s, parenteral nutrition (PN) has been the dominant mode for administering postoperative nutritional support. PN and enteral nutrition (EN) have been successfully used to treat varied severe diseases[1,2]. But in patients who are recovering from pancreatitis or who have a pancreatic fistula, efforts should be made to avoid stimulation of pancreatic secretion. More recent experimental studies and clinical findings about small intestinal motility and absorption, the development of needle-catheter jejunostomy, and the appearance of new diets have given impetus to the use of enteral feeding for early postoperative nutritional support and the use of octreotide to inhibit pancreatic fistula[3-5]. During application of enteral feeding, the question arises. How the pancreatic secretion was influenced by EN administered into the second jejunal loop? Can the more expensive and less physiological PN be replaced by this method[2]? Since the exocrine function of the pancreas is stimulated by the vagus nerve and the release of gastrointestinal hormones in response to food, there might be no significant increase in the exocrine activity of the pancreas. Animal experiments supported this supposition[6,7]; however, perfusion of distal jejunum with essential amino acids in human and infusion of carbohydrate into the ileum in human and dog enhanced pancreatic enzyme secretion. The present study was to evaluate the effect of parenteral and EN combined with octreotide on pancreatic secretion of the patients with pancreatic fistula by determining the levels of pancreatic secretion, amylase and electrolytes.
Seventeen patients (12 male and 5 female; mean age 43 years, range 27 to 71 years) with pancreatic fistula after abdominal injury or operation from July1997 to March 2003 were recruited and randomly divided into PN group (n = 9) and EN group (n = 8). It included pancreatic fistula after pancreas injury in 8 cases, pancreatic body and tail tumor resection in 5 cases, pancreatoduodenectomy in 4 cases.
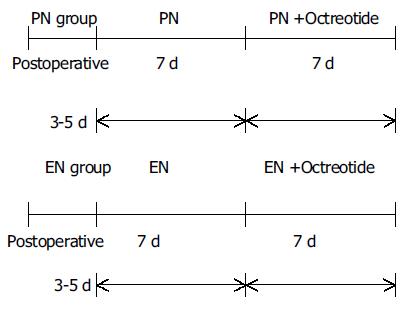
Nine cases with pancreatic fistula 3-5 d postoperatively were supported by PN for 2 wk. In the first wk, they received only PN. In the second week they received PN and octreotide. The target of nutritional therapy was to provide 20-25 kcal/(kg·d) non-protein diet and 0.12-0.15 g protein/(kg·d), the ratio of calories to nitrogen was (100-120):1.PN was given through continuous infusion (14 h/d) of a mixture of 200 mL/L Intralipid or 200 mL/L Lipofundin (B.Braun), 500 g/L glucose and 70 g/L Vamin and branched-chain amino acid. Seizure energy index of glucose to fat emulsion was 1:1. Multivitamins and electrolytes were also administered into total PN (TPN) solutions. Insulin was supplemented into PN at a ratio of glucose vs insulin 6:1. Six patients received TPN through right subclavian vein, 3 cases through right jugular vein. TPN was infused initially at a rate of 40 mL/h and was increased by 20 mL/h every 4 h. Antibiotics were infused by another peripheral vein. The liver and renal function, bicarbonate, serum glucose, fat metabolism were measured every other day. Human albumin, plasma and fresh blood were infused according to the patient’s condition. Octreotide 0.3 mg/500 mL saline was infused continuously for 8 h in the second wk and 0.1 mg octreotide was injected subcutaneously at 8:00 pm per day for 7 d.
Eight cases with pancreatic fistula were administered EN 3-5 d postoperatively. Nutrison (Nutricia) was used. Enteral feeding included 2 cases of nasoduodenal tube feeding, 4 cases of jejunectomy, and 2 cases of endoscopically placed nasojejunal tube. The jejunum through jejunostomy catheter was infused 250 mL Nutrison and 500 mL 9 g/L saline during days 1-3; Nutrison 500-1000 mL and 500 mL milk or vegetable soup were infused for 10-14 d. The infusion rate was controlled by a microcomputer-pump (Nutricia). During EN, inadequate calories and nitrogen were supplemented by partial PN. Octreotide 0.3 mg/500 mL saline was continuously infused for 8 h in the second week, 0.1 mg octreotide was injected subcutaneously at 8:00 pm per day for 7 d.
Pancreatic juice was collected on ice from the fistula during PN or EN and on d 1, 4, 7 before and after using octreotide, respectively. Samples were stored at -20 until amylase and protein content of the fractions were measured by established methods. Experimental procedures are shown as below.
Math 1
Data were collected by two persons blinded to the patients and presented as mean ± SD. Statistical significance was evaluated using t test by SPSS 10.0 statistical-software, and a P value less than 0.05 was considered significant.
Though pancreatic juice, protein and amylase in EN group were slightly increased as compared with PN group, there were no significant changes between the two groups at the first wk (P > 0.05). During the second wk, the pancreatic juice, protein, amylase decreased markedly from 79.6 mL/d to 60.8 mL/d, 31 mg/L to 22.8 mg/L and 4220 U/L to 3270 U/L, respectively, and there was a significant statistical difference as compared with that of pre-using octreotide in PN group (P < 0.05). During EN combined with octreotide, the pancreatic juice, protein, amylase secretion decreased significantly from 87.9 mL/d to 65.3 mL/d, 36 mg/L to 29.1 mg/L, and 4440 U/L to 3670 U/L, respectively, and there is a statistical difference as compared with that of pre-using octreotide (P < 0.05); but there was no statistical difference between two groups after using octreotide (P > 0.05) (Table 1).
| Group | n | Pancreatic juice (mL/d) | Protein (mg/L) | Amylase (U/L) | ||||||
| d 1 | d 4 | d 7 | d 1 | d 4 | d 7 | d 1 | d 4 | d 7 | ||
| PN | 9 | 86 ± 35 | 77 ± 49 | 81 ±31 | 34 ± 17 | 30 ± 17 | 29 ± 17 | 4 268 ± 1 361 | 4 026 ± 1 131 | 4 468 ± 1 487 |
| PN + Oc- treotide | 9 | 64 ± 21a | 58 ± 19a | 60 ±23a | 25 ± 17a | 21 ± 17a | 23 ± 17a | 3 268 ± 1 031a | 3 342 ± 1 116a | 3 081 ± 1 213a |
| EN | 8 | 91 ± 73 | 81 ± 73 | 93 ± 73 | 36 ± 24 | 37 ± 24 | 35 ± 24 | 4 761 ± 987 | 4 361 ± 1 023 | 4 276 ± 912 |
| EN + Oc- treotide | 8 | 67 ± 29c | 61 ± 39a | 66 ± 34a | 27 ± 12a | 31 ± 17 | 30 ± 10 | 3 963 ± 1 104a | 3 566 ± 1 001a | 3 363 ± 1 221a |
During the first wk, there were no differences in HCO3-, K+, Na+ and Cl- secretion in pancreatic juice between the two groups. After PN combined with octreotide, HCO3-, K+, Na+, and Cl-secretion decreased markedly, from 71 mmol/d to 45 mmol/d, 30 mmol/L to 21 mmol/L, 130 mmol/L to 87 mmol/L, and 60 mmol/L to 45 mmol/L, respectively, and there was a statistical difference as compared with that of pre-using octreotide, respectively (P < 0.05). During EN supporting, there were no differences in HCO3-, K+, Na+ and Cl- secretion in pancreatic juice as compared with PN. During the second wk, HCO3-, K+, Na+, and Cl- secretion in EN group decreased significantly, from 79 mmol/d to 52 mmol/d, 35 mmol/L to 25 mmol/L, 135 mmol/L to 94 mmol/L, and 74 mmol/L to 54 mmol/L, respectively, there was a statistical difference as compared with that of pre-using octreotide (P < 0.05); but there was no statistical difference between two groups after using octreotide (P > 0.05) (Table 2).
| Group | n | HCO3- | K+ | Na+ | Cl- | ||||||||
| d 1 | d 4 | d 7 | d 1 | d 4 | d 7 | d 1 | d 4 | d 7 | d 1 | d 4 | d 7 | ||
| PN | 9 | 73 ± 37 | 69 ± 31 | 71 ± 32 | 32 ± 19 | 28 ± 12 | 31 ± 17 | 123 ± 33 | 133 ± 31 | 126 ± 43 | 61 ± 34 | 63 ± 31 | 58 ± 24 |
| PN+Oct | 9 | 54 ± 34a | 57 ± 24a | 59 ± 31a | 22 ± 11a | 19 ± 9a | 21 ± 17a | 87 ± 53a | 78 ± 67a | 93 ± 55a | 49 ± 13a | 42 ± 21a | 49 ± 17a |
| EN | 8 | 79 ± 44 | 81 ± 35 | 83 ± 47 | 37 ± 17 | 36 ± 13 | 34 ± 19 | 131 ± 44 | 129 ± 33 | 139 ± 28 | 73 ± 37 | 71 ± 43 | 68 ± 31 |
| EN+Oct | 8 | 65 ± 23c | 61 ± 33c | 57 ± 37c | 25 ± 15c | 28 ± 11c | 28 ± 13 | 101 ± 63c | 95 ± 79c | 91 ± 73c | 51 ± 15c | 49 ± 21c | 53 ± 35c |
As many as 50%-70% of patients underwent enterocutaneous pancreatic fistulas after operation[5]. High-out fistulas (more than 200 mL daily) result in significant loss of fluid, alteration in acid-base status and malnutrition. In addition, digestive enzymes, often of extreme pH, can cause inflammation and soft tissue or skin destruction along the fistula tract. These increase the risk of infection and hemorrhage, prolong the time required for spontaneous fistula closure and increase postoperative mortality rates. Conservative management of enterocutaneous pancreatic fistula, including TPN, skin care and infection control, succeeds in a spontaneous closure rate of 24%-73%[5-10]. However, such treatment is often of long duration (2-3 mo) and high cost, and is associated with considerable morbidity. In cases in which maximal medical treatment has failed, re-operation for fistula closure is required.
In theory, reduction of fistula output should promote the chances of spontaneous closure. This is usually achieved by restriction of oral intake, sometimes supplemented with nasogastric suction in order to relieve the burden of gut and pancreas. Pharmacological attempts to decrease intestinal secretions involve using drugs such as somatostatin, loperamide, atropine or pirenzepine, and H2 blockers such as omeprazole. Although the effectiveness of such treatments has yet to be confirmed[7]. In clinics, PN or EN is usually considered, and they may play an important role in the spontaneous closure of pancreatic fistula, but there is some controversy over their effectiveness and safety.
TPN with complete gut rest has been proposed as adjuvant therapy in the treatment of severe acute pancreatitis, to meet metabolic demands and relieve pancreas. A decrease in volume of pancreatic secretion and, in particular, its enzymatic content would likely have a beneficial effect on patient’s clinical course. That TPN decreases pancreatic activity is supported by several animal studies. Hamilton et al documented a marked decrease in pancreatic secretions of the normal dog pancreas stimulated maximally by secretion and pancreozymin with the institution of TPN. The volume of pancreatic secretion decreased by 50% and amylase secretion decreased by 71%, but there was a slight increase in bicarbonate excretion. Adler, in 1975, demonstrated that intravenous administration of hypertonic solutions including 50% dextrose resulted in a 24% decrease in pancreatic secretion and a 69% decrease in biliary secretion in a maximally secretin- and pancreozymin-stimulated dog model. PN for patients with pancreatitis has, in general, shown no adverse or beneficial effects on the course of pancreatitis but has provided adequate nutritional support.
The use of intravenous lipid formulations in patients with pancreatic fistula remains controversial despite the evidence that they are not harmful. Kelly and Nahrwold[11] compared the exocrine pancreatic secretory response to intravenous saline and PN in dogs with chronic gastric fistula and found that the latter produced a small but significant increase in the mean volume of pancreatic juice (2.4 vs 1.8 mL per 15 min; P < 0.05) with a slight increase in mean protein and bicarbonate secretions. These differences are minimal compared with the changes found in dogs receiving an intraduodenal infusion of an enteral diet. Hamiloon reported that PN could ameliorate stimulation to the pancreas. The output of the pancreatic juice decreased by 50%, that of amylase by 71% and that of glucose slightly increased. Another canine with chronic pancreatic fistula and stomach ectomy received fat emulsion infusion. Meanwhile, the canine received stimulation of secretin. The results showed that pancreatic juice, protein enzyme, total protein and HCO3- did not change during infusion of fat emulsion[12]. Patients with pancreatic fistula postoperative of gastrectomy received PN by administration of 640 mL or 840 mL 100 g/L Liposym for 8 d, the pancreatic juice, protein and HCO3- did not increase[13]. Klein et al[14] believed that the effects of different PN contents on the pancreatic juice, HCO3- and amylase secretion were different. Intralipid could increase pancreatic juice from 33 mL/h to 39 mL/h, HCO3- from 44.5 mmol/L to 54.5 mmol/L, amylase from 25000 U/L to 32000 U/L. They held that fat emulsion should be used to supplement essential fatty acid. In the present study, 250 mL 200 mL/L Intralipid or 200 mL/L Lipofundin per day was used as fat energy sources for 2 wk. The results showed that pancreatic juice, protein, amylase, HCO3-, K+, Na+ and Cl- secretion did not change. It suggested that PN could not stimulate pancreatic secretion, and supplemental fat emulsion was safety.
The presence of food in the stomach and duodenum elicits gastropancreatic and duodenopancreatic reflexes that result in stimulation of pancreatic exocrine secretion. However, these effects are not pronounced when nutrients are delivered directly into jejunum.
A study[15] in dogs showed that intragastric delivery of nutrients caused an increase in the volume, and protein and bicarbonate content of pancreatic secretions compared with those in saline-infused controls. Intraduodenal feeding increased the volume of pancreatic secretions but did not affect protein or bicarbonate secretion. Other studies[16] in dogs also showed that the intraduodenal administration of elemental diets or pure amino acid solutions significantly increased pancreatic secretions, suggesting that the amino acid content of elemental diets is responsible for the stimulatory effects. In contrast, intrajejunal delivery of nutrients was not associated with a significant change in the volume, or protein or bicarbonate content of pancreatic secretions compared with controls. These authors also found in one human subject that avoidance of cephalic, gastric, and duodenal stimuli by jejunal tube feeding of acids did not result in pancreatic stimulation. Another canine study[17], in which an elemental diet was fed into the proximal jejunum, demonstrated a brisk pancreatic secretory response. However, the pancreatic juice collected was watery and low in enzyme. They concluded that bypassing the stomach minimized acid secretion, which played an important role in keeping the pancreas at rest. Bodoky et al[18] randomized 12 patients undergoing pylorus-preserving pancreaticoduodenectomy for chronic pancreatitis to receive EN via a needle catheter feeding jejunostomy (7 patients) or PN (5 patients). A catheter placed during operation in the pancreatic duct was used to collect pancreatic secretions. The authors believed that the disease was mainly in the pancreatic head and that the function of the pancreatic remnant was nearly normal. They did not find any difference in the volume of pancreatic secretions or the content of bicarbonate, protein, chymotrypsin or amylase between the two groups.
In the present study, 8 patients received EN for 2 wk, during which the volume of pancreatic juice, protein, amylase and bicarbonate in EN group had a small but not significant increase as compared with PN group. Although there is a paucity of human studies on the effects of oral feeding and EN on pancreatic secretion, one might reasonably conclude from available evidence from human and canine studies that oral, intragastric and intraduodenal feeding produce a significant stimulation of pancreatic secretions. In contrast, intrajejunal feeding has much fewer stimulatory effects and is therefore the route of choice for enteral feeding in pancreatic diseases.
Discovered in 1972, somatostatin is a naturally occurring tetradecapeptide with a wide spectrum of inhibitory biological actions. Within the gastrointestinal tract, somatostatin has been found in the pancreas, stomach, intestinal mucosa and mesenteric neurons. Because of its inhibitory actions, it has been used in the management of upper gastrointestinal haemorrhage, secretory diarrhoea, dumping syndrome and peptide-secreting tumors. Since the mid-1980 s, somatostatin has been advocated as an adjuvant therapy in the conservative treatment of patients with enterocutaneous pancreatic fistula[19,20]. However, its short half-life (1.1-3.0 min) mandates continuous administration. Long-acting somatostatin analogues, such as octreotide acetate and lanreotide, have been developed. Octreotide has a biological half-life of 90-120 min and can be administered subcutaneously two or three times per day. Compared with somatostatin, it not only significantly reduces the secretions of the pancreas and stomach, but also has a more selective range of targets. Both somatostatin and octreotide have been described as effective treatments for enteric fistulas in adults, with few severe short-term side-effects (mostly diarrhoea, flushing and abdominal pain). These preparations have, therefore, the potential benefit of cutting the cost of treatment and reducing the discomfort of patients by shortening the length of hospital stay. Since 1990, several groups have described favourable results using somatostatin or octreotide for the “conservative” management of enterocutaneous pancreatic fistula. Administration of such drugs has been reported to result in rapid and significant reduction of fistula output (often within 24 h of treatment) and acceleration of fistula closure (from as early as day 2)[21-25].
Martineau et al[4] reviewed the role of octreotide for the management of established enterocutaneous pancreatic fistulas by summarizing the results of 4 randomized trials and 8 case series. They concluded that octreotide significantly decreased enterocutaneous pancreatic fistula output, but did not significantly improve the rate of spontaneous fistula closure. Furthermore, octreotide did not seem to reduce the output, or hasten the time or rate of closure, in fistula of recent onset (less than 8 d). Recently, Berberat et al[8] reviewed six randomized controlled trials and one open randomized trial, and assessed the efficacy of octreotide on preventing postoperative complications in patients underwent major pancreatic operations. They concluded that prophylactic use of octreotide could significantly reduce the complication rate. Sitges-Serra[9] reported that 20 cases with pancreatic fistula received octreotide 10 μg/8 h for 20 d (n = 13). The output of pancreatic juice decreased from 725 mL/24 h to 151 mL/24 h, the rate of spontaneous fistula closure was 78%. Another group (n = 7) used octreotide 100 μg/8 h for 7 d. The output of pancreatic juice increased from 218 mL/24 h to 436 mL/24 h. Torres[26] reported 33 patients with pancreatic fistula received octreotide 75-100 μg/8 h for 10 d, and then stopped. It resulted in increase of pancreatic juice secretion from 228 mL/24 h to 498 mL/24 h. After 6 h, octreotide was used again. Output of pancreatic juice decreased from 828 mL/24 h to 247 mL/24 h, the rate of spontaneous fistula closure was 79%. The time of spontaneous fistula closure of octreotide group was 2-10 d shorter than that of control group. In the present study, the patients with pancreatic fistula received octreotide combined with PN and EN, the secretion of pancreatic juice, protein and amylase was decreased, and secretion of HCO3-, K+, Na+ and Cl- was inhibited. These results showed that octreotide can inhibit pancreatic juice, amylase secrete, and maybe beneficial to pancreatic fistula self-repair.
We conclude that administration of nutrient solutions into second jejunal loop does not overstimulate the exocrine function of pancreas, while it produces equivalent results to parenteral administration of nutrients. The enteral feeding combined with octreotide could be effective and safe to reduce pancreatic fistula output.
Edited by Zhu LH and Chen WW Proofread by Xu FM
| 1. | Kalfarentzos FE, Karavias DD, Karatzas TM, Alevizatos BA, Androulakis JA. Total parenteral nutrition in severe acute pancreatitis. J Am Coll Nutr. 1991;10:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, Eckhert CD, Hollander D, Gornbein J, Kopple JD, Vijayaroghavan SR. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995;19:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 274] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Dorta G. Role of octreotide and somatostatin in the treatment of intestinal fistulae. Digestion. 1999;60 Suppl 2:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Martineau P, Shwed JA, Denis R. Is octreotide a new hope for enterocutaneous and external pancreatic fistulas closure? Am J Surg. 1996;172:386-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | De Graef J, Woussen-Colle MC. [Physiological control and pharmacological inhibition of digestive secretions]. Acta Chir Belg. 1985;85:149-153. [PubMed] |
| 6. | Qin HL, Su ZD, Hu LG, Ding ZX, Lin QT. Effect of early intrajejunal nutrition on pancreatic pathological features and gut barrier function in dogs with acute pancreatitis. Clin Nutr. 2002;21:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Qin HL, Su ZD, Hu LG, Ding ZX, Lin QT. Parenteral versus early intrajejunal nutrition: effect on pancreatitic natural course, entero-hormones release and its efficacy on dogs with acute pancreatitis. World J Gastroenterol. 2003;9:2270-2273. [PubMed] |
| 8. | Berberat PO, Friess H, Uhl W, Büchler MW. The role of octreotide in the prevention of complications following pancreatic resection. Digestion. 1999;60 Suppl 2:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Sitges-Serra A, Guirao X, Pereira JA, Nubiola P. Treatment of gastrointestinal fistulas with Sandostatin. Digestion. 1993;54 Suppl 1:38-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Voss M, Ali A, Eubanks WS, Pappas TN. Surgical management of pancreaticocutaneous fistula. J Gastrointest Surg. 2003;7:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Kelly GA, Nahrwold DL. Pancreatic secretion in response to an elemental diet and intravenous hyperalimentation. Surg Gynecol Obstet. 1976;143:87-91. [PubMed] |
| 12. | Burns GP, Stein TA. Pancreatic enzyme secretion during intravenous fat infusion. JPEN J Parenter Enteral Nutr. 1987;11:60-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Edelman K, Valenzuela JE. Effect of intravenous lipid on human pancreatic secretion. Gastroenterology. 1983;85:1063-1066. [PubMed] |
| 14. | Klein E, Shnebaum S, Ben-Ari G, Dreiling DA. Effects of total parenteral nutrition on exocrine pancreatic secretion. Am J Gastroenterol. 1983;78:31-33. [PubMed] |
| 15. | Ragins H, Levenson SM, Signer R, Stamford W, Seifter E. Intrajejunal administration of an elemental diet at neutral pH avoids pancreatic stimulation. Studies in dog and man. Am J Surg. 1973;126:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 85] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Wolfe BM, Keltner RM, Kaminski DL. The effect of an intraduodenal elemental diet on pancreatic secretion. Surg Gynecol Obstet. 1975;140:241-245. [PubMed] |
| 17. | Cassim MM, Allardyce DB. Pancreatic secretion in response to jejunal feeding of elemental diet. Ann Surg. 1974;180:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 18. | Bodoky G, Harsanyi L, Pap A, Tihanyi T, Flautner L. Effect of enteral nutrition on exocrine pancreatic function. Am J Surg. 1991;161:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Gray M, Jacobson T. Are somatostatin analogues (octreotide and lanreotide) effective in promoting healing of enterocutaneous fistulas? J Wound Ostomy Continence Nurs. 2002;29:228-233. [PubMed] |
| 20. | Voss M, Pappas T. Pancreatic Fistula. Curr Treat Options Gastroenterol. 2002;5:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Li-Ling J, Irving M. Somatostatin and octreotide in the prevention of postoperative pancreatic complications and the treatment of enterocutaneous pancreatic fistulas: a systematic review of randomized controlled trials. Br J Surg. 2001;88:190-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Takács T, Hajnal F, Németh J, Lonovics J, Pap A. Stimulated gastrointestinal hormone release and gallbladder contraction during continuous jejunal feeding in patients with pancreatic pseudocyst is inhibited by octreotide. Int J Pancreatol. 2000;28:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Duerksen DR, Bector S, Yaffe C, Parry DM. Does jejunal feeding with a polymeric immune-enhancing formula increase pancreatic exocrine output as compared with TPN? A case report. Nutrition. 2000;16:47-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Stabile BE, Borzatta M, Stubbs RS. Pancreatic secretory responses to intravenous hyperalimentation and intraduodenal elemental and full liquid diets. JPEN J Parenter Enteral Nutr. 1984;8:377-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Grant JP, Davey-McCrae J, Snyder PJ. Effect of enteral nutrition on human pancreatic secretions. JPEN J Parenter Enteral Nutr. 1987;11:302-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Torres AJ, Landa JI, Moreno-Azcoita M, Argüello JM, Silecchia G, Castro J, Hernandez-Merlo F, Jover JM, Moreno-Gonzales E, Balibrea JL. Somatostatin in the management of gastrointestinal fistulas. A multicenter trial. Arch Surg. 1992;127:97-9; discussion 100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 2.6] [Reference Citation Analysis (0)] |