Published online Aug 15, 2004. doi: 10.3748/wjg.v10.i16.2379
Revised: February 14, 2004
Accepted: March 2, 2004
Published online: August 15, 2004
AIM: To determine the efficacy and safety of DA-9601 on erosive gastritis versus cetraxate as a standard drug by gastrointestinal endoscopy.
METHODS: Five hundred and twelve patients with erosive gastritis were divided into three groups. The groups received 180 mg or 360 mg of DA-9601, or 600 mg of cetraxate (NeuerTM) t.i.d. for 2 wk, respectively. Endoscopic observations were performed before and 2 wk after the treatment, and the cure and improvement rates were investigated.
RESULTS: Of the 512 intention-to-treat (ITT) population, 457 patients comprised the per protocol (PP) analysis. Endoscopic cure rate was significantly higher in the DA-9601 group than in the cetraxate group in both the PP (56%, 58% vs 36%; DA-9601 180 mg, 360 mg and cetraxate, respectively) and ITT (52%, 51% vs 35%) populations. Two DA-9601 groups (180 and 360 mg) had significantly higher endoscopic improvement rates than the cetraxate group in both the PP (67%, 65% vs 46%) and ITT (63%, 58% vs 45%) populations. The percentage of symptom relief over the 2 wk was found not significantly different between groups. During the study, both DA-9601 and cetraxate produced no treatment-associated adverse events.
CONCLUSION: From these results, it appears that DA-9601 has excellent efficacy on erosive gastritis. This study also confirms the safety profile of DA-9601.
- Citation: Seol SY, Kim MH, Ryu JS, Choi MG, Shin DW, Ahn BO. DA-9601 for erosive gastritis: Results of a double-blind placebo-controlled phase III clinical trial. World J Gastroenterol 2004; 10(16): 2379-2382
- URL: https://www.wjgnet.com/1007-9327/full/v10/i16/2379.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i16.2379
Gastritis is a heterogeneous pathological condition, and is one of the most frequent reasons for medical consultation in Asian countries, including Korea and Japan. However, in Western countries, these conditions are diagnosed as non-ulcer dyspepsia (NUD), which affects approximately one in five Americans[1-4]. Gastritis, the “precursor” lesion to mucosal ulceration is both an important clinical entity and an important cause of abdominal pain in children[5]. Inflammation of the gastric mucosa is the end result of an imbalance between mucosal defensive and aggressive factors (i.e., disturbances in gastric acidity and the mucus-bicarbonate barrier), and recently a great deal of attention has been focused on gastric hormones, specifically gastrin, and pepsinogens I and II[6,7].
Gastritis can be classified into acute or chronic forms based upon Sydney System, and chronic gastritis can be subclassified as nonatrophic, atrophic, and special types[8,9]. Using this Sydney pathologic classification as a guide, Chen et al.[10] reported a simplified classification for gastritis based on practical radiologic evaluation, including erosive gastritis (acute and chronic), Helicobacter pylori (H pylori) gastritis, chronic nonspecific gastritis, hyperplastic gastritis, and miscellaneous types (including granulomatous, phlegmonous, eosinophilic, corrosive, other infectious types and rare types).
At present, the exact pathophysiology of this syndrome is poorly understood. However, current evidence suggests that H pylori infection, changes in lifestyles, eating behaviors, and nonsteroidal anti-inflammatory drug (NSAID) ingestion are causative factors in the pathogenesis of gastric mucosal injury in humans[11]. H pylori is known to be a particularly important pathogen in gastric and duodenal inflammation by producing excessive mucosal-reactive oxygen species (ROS), which damage the cell membrane and deplete gastric antioxidants[12].
The current rationale for drug treatment in gastritis is similar to other gastrointestinal disorders (eg., non-ulcer dyspepsia), and depends mainly on symptomatic relief using gastroprotective agents (e.g., rebamipide, teprenone, ecabet sodium, sofalcone, cetraxate, etc.), H2 receptor antagonists (e.g., cimetidine, ranitidine, famotidine, etc.), and antacids[13]. However, despite many efforts, the pharmacological treatment of patients with gastritis usually achieves only partial symptomatic relief in the majority of cases.
DA-9601 (StillenTM), a phytopharmaceutical derived from Artemisia asiatica, has been reported to possess antioxidative and cytoprotective actions in various models of gastric mucosal damage[14-16]. Though the mode of action of DA-9601 has not been fully elucidated, this new antioxidative drug scavenges superoxide and hydroxyl radicals, possesses potent anti-inflammatory activities, regenerates mucosal epithelial cells, and enhances the cytoprotective cytokines[17-19].
The present study was designed to assess the therapeutic effects and safety of DA-9601 on gastritis. Cetraxate, which has been shown to have therapeutic effects on gastritis was selected as the standard drug for comparative purposes.
The patients examined in this study consisted of 550 erosive gastritis patients who were diagnosed endoscopically between August 2000 and December 2001. All patients gave their written consent to this study. Out of 550 initially enrolled patients, 512 completed the study. Those 512 patients were subsequently randomized, of whom 326 were allocated to DA-9601 (186 had 180 mg and 140 had 360 mg daily) and 186 to cetraxate 600 mg. The exclusion criteria were: a history of peptic ulcer disease and reflux esophagitis; the presence of a malignant tumor in the digestive tract; the use of drugs capable of interfering with digestive mucosal integrity, gastric secretion or gastrointestinal motility, including H2 receptor antagonists, NSAIDs, muscarinic antagonists, gastroprotective agents (within the previous 14 d); thrombotic patients (cerebral thrombosis, myocardial infarction, thrombophlebitis, etc.); consumption coagulopathy patients; a history of hypersensitivity to drugs (rash, fever, itching, etc.); the presence of major hematological, renal, cardiac, pulmonary, or hepatic abnormalities.
The study was performed as a randomized, double-blind, placebo-controlled, multicenter trial. Subjects were recruited at the following five Korean centers; Inje University of Medicine (Busan), Ulsan University of Medicine (Seoul), Chunnam University of Medicine (Gwangju), Catholic University of Medicine (Seoul), and Ajou University of Medicine (Suwon). Gastrointestinal endoscopies were performed in all patients before starting therapy, and 2 wk later. Patients diagnosed with erosive gastritis were included and divided into 3 groups following initial symptom assessment, and either treated with DA-9601 180 or 360 mg, or cetraxate 600 mg t.i.d. for 2 wk. After completing the therapy, endoscopic examinations were conducted according to the following grades: score 1, no erosions; score 2 (mild), erosion number between 1 and 2; score 3 (moderate), erosion number between 3 and 5; score 4 (severe), erosion number more than 6, for the evaluation of cure rate and improvement rate (Table 1). In addition, all patients completed a standardized subjective assessment questionnaire, according to Table 2. Safety surveillance was done at the end of therapy, based on responses to a complaint questionnaire, results of physical examinations and laboratory tests. Blood samples were obtained at the end of therapy to determine concentrations of GPT, GOT, and bilirubin. Complaint questionnaires were recorded for the appreciable harmful or unpleasant reactions experienced by a patient as a result of drug therapy.
| Score | Number of erosions |
| 1 (none) | 0 |
| 2 (mild) | 1–2 |
| 3 (moderate) | 3–5 |
| 4 (severe) | 6 < |
| Score | Criteria |
| 1 (none) | No symptom |
| 2 (weak) | Symptoms occurred once a week |
| 3 (moderate) | The symptoms did not affect their life and occurred more than twice a week |
| 4 (strong) | The symptoms affected their everyday life and occurred daily |
Endoscopic efficacy was analyzed on an intention-to-treat (ITT) and per protocol (PP) basis. Both subjective and objective criteria were analyzed using Duncan’s multiple test. Comparisons between treatment groups were performed using the chi-square test. Data were considered to be significant when P < 0.05.
A total of 512 patients completed the trial. Baseline characteristics of the study populations are detailed in Table 3, which shows that the study groups were comparable with respect to demographics and disease-specific characteristics.
| Characteristic | DA-9601 180 mg (n = 186) | DA-9601 360 mg (n = 140) | Cetraxate 600 mg (n = 186) |
| Male sex | 89 (47.9) | 72 (51.4) | 92 (49.5) |
| Age(yr) | 45.9 ± 11.2 | 44.6 ± 12.1 | 46.4 ± 11.5 |
| History < 1 wk | 3 (1.6) | 1 (0.7) | 3 (1.6) |
| 1–4 wk | 31 (16.7) | 28 (20.0) | 29 (15.6) |
| > 4 wk | 98 (52.7) | 27 (19.3) | 97 (52.2) |
| Unknown | 54 (29.0) | 84 (60.0) | 57 (30.7) |
| Type Erosion | 186 (100) | 140 (100) | 186 (100) |
| Bleeding | 8 (4.3) | 4 (2.9) | 10 (5.4) |
| Redness | 51 (27.4) | 47 (33.6) | 44 (23.7) |
| Edema | 2 (1.1) | 10 (7.1) | 5 (2.7) |
| Grade Mild | 14 (7.5) | 18 (12.9) | 21 (11.3) |
| Moderate | 50 (26.9) | 40 (28.6) | 54 (29.0) |
| Severe | 122 (65.6) | 82 (58.6) | 111 (59.7) |
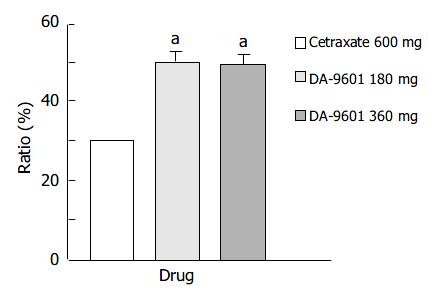
The ITT population was composed of 512 patients (186 in the DA-9601 180 mg group, 140 in the DA-9601 360 mg group, and 186 in the cetraxate 600 mg group) (Table 4). Endoscopic cure rates in DA-9601 180 mg, 360 mg, and cetraxate 600 mg groups were 52.2%, 51.4%, and 35%, respectively (Figure 1). A significant difference was found between the DA-9601 and cetraxate groups (P < 0.05), however, no difference in cure rates was found between the DA-9601 groups.
| Number of patients | |||
| DA-9601 180 mg | DA-9601 360 mg | Cetraxate 600 mg | |
| ITT analysis | 186 | 140 | 186 |
| PP analysis | 171 | 120 | 166 |
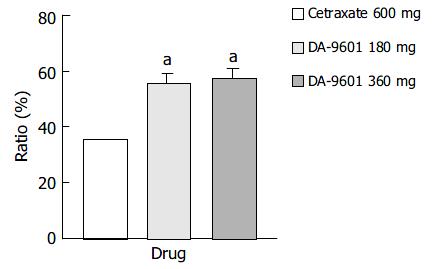
The PP assay population comprised 457 patients (171 in the DA-9601 180 mg group, 120 in the DA-9601 360 mg group, and 166 in the cetraxate 600 mg group) (Table 4). The estimated cure rates of erosive gastritis by PP analysis in DA-9601 180 mg, 360 mg, and cetraxate 600 mg groups were 55.6% (95/171), 57.5% (69/120), and 35.5% (59/166), respectively (Figure 2). The cure rates between the DA-9601 and cetraxate groups (P < 0.05) were significantly different, however, no difference was found between the DA-9601 groups.
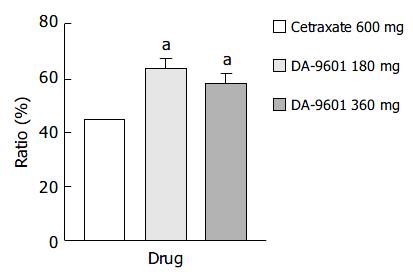
Estimated improvement rates by ITT analysis of erosive gastritis patients treated with cetraxate (600 mg) and DA-9601 (180 or 360 mg) showed statistically significant differences (P < 0.05); however, no difference was observed between the DA-9601 treated populations (Figure 3). The improvement rates of those treated with DA-9601 180 mg, 360 mg and cetraxate 600 mg were 63.4% (118/186), 57.9% (81/140), and 44.6% (83/186), respectively.
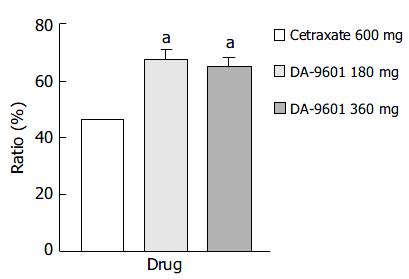
The estimated improvement rates by PP analysis for erosive gastritis treated with DA-9601 or cetraxate were statistically different (P < 0.05) (Figure 4). However, no difference was found between the DA-9601 treated groups. The estimated improvement rates were 67.3% (115/171), 65.0% (78/120), and 46.4% (77/166) in the DA-9601 180 mg, DA-9601 360 mg, cetraxate 600 mg treated groups.
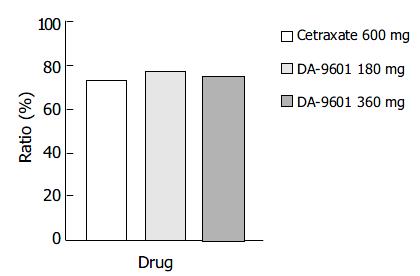
The symptom relief rates determined by the ITT and PP methods showed no statistically significant difference between the study populations. The overall degrees of symptom reduction by the ITT method in the DA-9601 180 mg, 360 mg, and cetraxate 600 mg groups were 77.4% (144/186), 75.0% (105/140), and 73.1% (136/186), respectively (Figure 5), and by PP analysis these were 81.3% (139/171), 81.7% (98/120), and 76.5% (127/166).
Serious adverse reactions were not encountered during this study. Of the 186 cetraxate 600 mg treated patients, the incidence of adverse effects was 7.5% (11 cases); including heartburn, abdominal pain, acid reflux, headache, itching, skin redness, facial edema, etc. Mild adverse events were observed in 14 of 186 patients treated with DA-9601 180 mg (Table 5). In the case of DA-9601 360 mg group, no notable change was observed either in subjective assessment questionnaire or in clinical examination at the end of the study as compared to DA-9601 180 mg or cetraxate groups. No statistically significant difference was observed between the cetraxate and DA-9601 treated groups.
| DA-9601 180 mg | Cetraxate 600 mg | |
| Gastrointestinal | ||
| Dyspepsia | 2 (1.08) | 0 |
| Nausea | 2 (1.08) | 0 |
| Diarrhea | 2 (1.08) | 0 |
| Heartburn | 1 (0.54) | 1 (0.54) |
| Abdominal pain | 1 (0.54) | 1 (0.54) |
| Acid reflux | 0 | 1 (0.54) |
| Vomiting | 1 (0.54) | 1 (0.54) |
| CNS and ANS | ||
| Dizziness | 1 (0.54) | 0 |
| Headache | 1 (0.54) | 1 (0.54) |
| Skin | ||
| Itching | 1 (0.54) | 1 (0.54) |
| Skin redness | 1 (0.54) | 1 (0.54) |
| Facial edema | 0 | 1 (0.54) |
| Liver | ||
| sGPT elevation | 1 (0.54) | 1 (0.54) |
| sGOT elevation | 0 | 1 (0.54) |
| Bilirubin elevation | 0 | 1 (0.54) |
| Total | 14 (7.53) | 11 (5.91) |
Although gastritis is the most common complication in the digestive tract, the definition of gastritis is not consensual due to differences in diagnostic criteria. It has been postulated that gastritis contributes to the natural history of ulcer development due to the decreased protection offered by the mucosal barrier lining the stomach, and increased epithelial cell exposure to hydrochloric acid.
The aim of this study was to demonstrate the efficacy and safety of DA-9601 versus cetraxate, a widely used anti-ulcer drug, for the treatment of erosive gastritis. Cetraxate, which has a mucosal protective effect, was first introduced in 1976 as an anti-ulcer drug[20]. It is widely used clinically in Japan and other Asian countries. The mode of action of cetraxate is ascribed to an increased gastric mucosal blood flow through enhanced nitric oxide synthase activity, and the prevention of a decrease in the mucosal prostaglandin content[21]. The results of the present randomized, double-blind, placebo-controlled, multicenter study demonstrate that orally administrated DA-9601 is both effective and well-tolerated in the treatment of erosive gastritis of various etiologies.
Adverse reactions were seen in 7.5% (14/186) of the patients in DA-9601 180 mg treated group and in 5.9% (11/186) of patients in the cetraxate 600 mg treated group. In both groups, the main adverse reactions were vomiting, abdominal pain, headache, skin rash, itching, and sGPT elevation. However, in DA-9601 360 mg treated group, no obvious adverse reactions were observed (0%, 0/140). Therefore, we concluded that there was no drug treatment related adverse reaction based on the lack of dose-response relationship. In the previous four-wk oral toxicity study of DA-9601 in rats at doses of 120, 500, and 2000 mg/kg.d, no treatment related alternations, including changes in blood biochemistry were observed[22]. In animal experiments, DA-9601 prevented acetaminophen, and CCl4-induced hepatic GSH depletion and CCl4-induced increased hepatic MDA (a parameter of lipid peroxidation) in a dose-dependent manner[23,24]. In addition, sGPT and sGOT levels showed a tendency to fall to below the normal range in DA-9601 treated patients (unpublished data).
Conclusively, the present study indicates that DA-9601 has an excellent efficacy and safety profile. Therefore, we believe that DA-9601 is a highly attractive option for the treatment of erosive gastritis, in which a balance between aggressive and defensive factors plays a significant role.
Edited by Zhu LH and Xu FM
| 1. | Talley NJ. Review article: dyspepsia: how to manage and how to treat? Aliment Pharmacol Ther. 2002;16 Suppl 4:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Talley NJ. Therapeutic options in nonulcer dyspepsia. J Clin Gastroenterol. 2001;32:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Talley NJ, Stanghellini V, Heading RC, Koch KL, Malagelada JR, Tytgat GN. Functional gastroduodenal disorders. Gut. 1999;45 Suppl 2:II37-II42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 415] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Kurata JH, Haile BM. Epidemiology of peptic ulcer disease. Clin Gastroenterol. 1984;13:289-307. [PubMed] |
| 5. | Blecker U, Gold BD. Gastritis and peptic ulcer disease in childhood. Eur J Pediatr. 1999;158:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Taylor IL. Gastrointestinal hormones in the pathogenesis of peptic ulcer disease. Clin Gastroenterol. 1984;13:355-382. [PubMed] |
| 7. | Defize J, Meuwissen SG. Pepsinogens: an update of biochemical, physiological, and clinical aspects. J Pediatr Gastroenterol Nutr. 1987;6:493-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3553] [Article Influence: 122.5] [Reference Citation Analysis (3)] |
| 9. | Whitehead R. The classification of chronic gastritis: current status. J Clin Gastroenterol. 1995;21 Suppl 1:S131-S134. [PubMed] |
| 10. | Chen MY, Ott DJ, Clark HP, Gelfand DW. Gastritis: classification, pathology, and radiology. South Med J. 2001;94:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 11. | Yoshikawa T, Naito Y. The role of neutrophils and inflammation in gastric mucosal injury. Free Radic Res. 2000;33:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Kashiwagi H. Ulcers and gastritis. Endoscopy. 2003;35:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Talley NJ. Update on the role of drug therapy in non-ulcer dyspepsia. Rev Gastroenterol Disord. 2003;3:25-30. [PubMed] |
| 14. | Oh TY, Ryu BK, Yang JI, Kim WB, Park JB, Oh TY, Lee SD, Lee EB. Studies on antiulcer effects of DA-9601, an Artemisia herba extract against experimental gastric ulcers and its mechanism. J Appl Pharmacol. 1996;4:111-121. |
| 15. | Lee EB, Kim WB, Ryu BK, Ahn BO, Oh TY, Kim SH. Studies on protective effect of DA-9601, an Artemisiae Herba extract, against ethanol-induced gastric mucosal damage and its mechanism. J Appl Pharmacol. 1997;5:202-210. |
| 16. | Oh TY, Ryu BK, Ko JI, Ahn BO, Kim SH, Kim WB, Lee EB, Jin JH, Hahm KB. Protective effect of DA-9601, an extract ofArtemisiae Herba, against naproxen-induced gastric damage in arthritic rats. Arch Pharm Res. 1997;20:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Lee JJ, Han BG, Kim MN, Chung MH. The inhibitory effect of eupatilin on Helicobacter pylori-induced release of leukotriene D4 in the human neutrophils and gastric mucosal cells. Korean J Physiol Pharmacol. 1997;1:573-580. |
| 18. | Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Lee KM, Hahm KB. Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 2001;49:364-371. [RCA] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Hahm KB. Oxidative damages are critical in pathogenesis of reflux esophagitis: implication of antioxidants in its treatment. Free Radic Biol Med. 2001;30:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Suzuki Y, Hayashi M, Ito M, Yamagami I. Anti-ulcer effects of 4'-(2-carboxyetyl) phenyl trans-4-aminomethyl cyclohexanecarboxylate hydrochloride (cetraxate) on various experimental gastric ulcers in rats. Jpn J Pharmacol. 1976;26:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Tachi K, Goto H, Hayakawa T, Sugiyama S. Prevention of water immersion stress-induced gastric lesions through the enhancement of nitric oxide synthase activity in rats. Aliment Pharmacol Ther. 1996;10:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Kim OJ, Kang KK, Kim DH, Baik NG, Ahn BO, Kim WB, Yang I. Four-week oral toxicity study of DA-9601, an antiulcer agent of Artemisia spp. Extract, in rats. J Appl Pharmacol. 1996;4:354-363. |
| 23. | Ryu BK, Ahn BO, Oh TY, Kim SH, Kim WB, Lee EB. Studies on protective effect of DA-9601, Artemisia asiatica extract, on acetaminophen- and CCl4-induced liver damage in rats. Arch Pharm Res. 1998;21:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Cheong JY, Oh TY, Lee KM, Kim DH, Ahn BO, Kim WB, Kim YB, Yoo BM, Hahm KB, Kim JH. [Suppressive effects of antioxidant DA-9601 on hepatic fibrosis in rats]. Taehan Kan Hakhoe Chi. 2002;8:436-447. [PubMed] |