Published online Aug 1, 2004. doi: 10.3748/wjg.v10.i15.2292
Revised: December 3, 2003
Accepted: December 16, 2003
Published online: August 1, 2004
AIM: To observe the effect of octreotide on apoptosis rate of human pancreatic cancer cells PC-3 after transfected with somatostatin receptor type 2 (SST2) gene.
METHODS: SST2 plasmid was transfected into PC-3 cells by liposome. Result of transfection was detected by immunocytochemical staining and Western blotting. Apoptosis rates of PC-3 cells under different dosages of octreotide were measured by MTT assay and flow cytometry (FCM).
RESULTS: Apoptosis rate caused by octreotide of transfected PC-3 cells was 7.56 ± 1.06% at the dosage of 0.20 μg/mL, 9.25 ± 1.73% at the dosage of 0.40 μg/mL and 14.18 ± 2.71% at the dosage of 0.80 μg/mL. Apoptosis rate caused by octreotide of non -transfected PC-3 cells was 5.76 ± 0.75% at the dosage of 0.20 μg/mL, 6.69 ± 0.80% at the dosage of 0.40 μg/mL and 7.26 ± 1.28% at the dosage of 0.80 μg/mL. Transfected PC-3 cells growth inhibition rate caused by octreotide was 9.36 ± 1.34% at the dosage of 0.20 μg/mL, 12.03 ± 1.44% at the dosage of 0.40 μg/mL and 20.23 ± 4.21% at the dosage of 0.80 μg/mL. Non-transfected PC -3 cells growth inhibition rate caused by octreotide was 6.44 ± 0.66% at the dosage of 0.20 μg/mL, 7.65 ± 0.88% at the dosage of 0.40 μg/mL and 9.29 ± 1.32% at the dosage of 0.80 μg/mL. We found that octreotide caused higher apoptosis rate and inhibition rate in transfected groups than in non-transfected groups (P < 0.05) at the tested dosages (0.20, 0.40 and 0.80 μg/mL).
CONCLUSION: Deficiency of SST2 was probably the major reason why octreotide had little effect on PC-3 cells. Transfecting SST2 gene could strengthen the ability of octreotide of killing PC-3 cells. It provided an experimental evidence for using both octreotide and transfection with SST2 gene on clinical treatment of pancreatic cancer.
- Citation: Liu ZR, Qin RY, Wu GS, Chang Q, Wang DY, Zou SQ, Qiu FZ. Effect of octreotide on human pancreatic cancer cells after transfected with somatostatin receptor type 2 gene. World J Gastroenterol 2004; 10(15): 2292-2294
- URL: https://www.wjgnet.com/1007-9327/full/v10/i15/2292.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i15.2292
Octreotide, the artificial synthetic somatostatin analogue, which possesses the advantage of relative specificity, long lasting and small ill effect, has already been applied to clinical therapy extensively. Its anti-tumor mechanism mainly depends on the expression of somatostatin receptor, especially SST2[1-3]. Most tumors highly express SST2, but some do not express or low express. For example, 90% human pancreatic cancer cells have lost the ability of expressing SST2, which leads to the unsatisfactory treatment effect in pancreatic cancer[4]. We did the experiment to explore octreotide’s effect on the apoptosis rate of pancreatic cancer (PC-3) cells after transfected with SST2 gene.
PC-3 cells were a gift from China Medical University. RPMI 1640, fetal calf serum, Liposome 2000 Reagent were purchased from Gibco Co. USA. Octreotide was kindly presented by Novartis Co. Swiss. MTT, DMSO and G418 were purchased from Sigma Co. USA. Goat-anti-human SST2 monoclonal antibody was purchased from Santa Cruz Co. USA. Rabbit-anti-goat and DAB were purchased from Zhongshan Biotechnology Co. Beijing, China. ECL kit was purchased from Amersham Pharmacia Biotechnology Inc. ABC test kit was purchased from Huamei Co. China. Human SST2 plasmid was kindly presented by Junbo Hu of Maryland University, USA.
SST2 plasmid amplification and transfection We adopted colon bacillus (DH-5α ) to amplify and extract the human SST2 plasmid, which was identified by endonuclease. We cultured the PC-3 cells with RPMI 1640 supplemented with 100 mL/L fetal calf serum. When PC-3 cells were in exponential growth phase, the human SST2 plasmid was transfected into PC-3 cells mediated by Liposome 2000 reagent and screened with culture medium with 600 mg/L G418.
Immunocytochemical staining Transfective effect was detected by immunocytochemical staining. ABC test kit was adopted to stain the cells crawling on the slides with immunocytochemical staining. Goat-anti-human SST2 monoclonal antibody was diluted to 1:100. Blank groups used PBS instead of goat-anti-human antibody.
Western blotting Expression of SST2 was detected by western-blot. Transfected cells were collected and lysed with the cell-lysis to extract protein. Goat-anti-human SST2 monoclonal antibody (1:1000) was used. The signals were developed with the ECL kit.
FCM Apoptosis rate was detected by flow cytometry. When the PC-3 cells were in exponential growth phase, octreotide at different dosages (0.05, 0.10, 0.20, 0.40, 0.80 μg/mL) was added. After 24 h, culture medium was renewed and octreotide added according to the above dosages. Cells in the culture medium were collected and preserved in 700 mL/L alcohol at -20 °C. Cells were collected after 48 h; the apoptosis rate was detected by flow cytometry.
MTT assay MTT assay was adopted to detect the sensitivity of PC-3 cells in vitro after using different dosages of octreotide. We divided them into three groups: blank groups (no cells), control groups (no drug) and octreotide groups. Octreotide treated cells were divided into five groups according to the dosages (0.05, 0.10, 0.20, 0.40, 0.80 µg/mL). Then the transfected PC-3 cells and non-transfected cells were inoculated into the 96-well culture panel, about 3×106/L in each well. Each panel had 10 replicate wells. After 24-h culture, octreotide was added and then cultivated for another 48 h. The sensitivity of PC-3 cells was detected with MTT assay and inhibition rate was measured by detecting the value of the A (λ = 550 nm). Inhibition rate of PC-3 cells growth = [(Acontrol group-A blank group) - (Aexperiment group-Ablank group)]/(Acontrol group-Ablank group) × 100%.
t-test and SPSS software were employed to analyze data. P < 0.05 indicates significant difference.
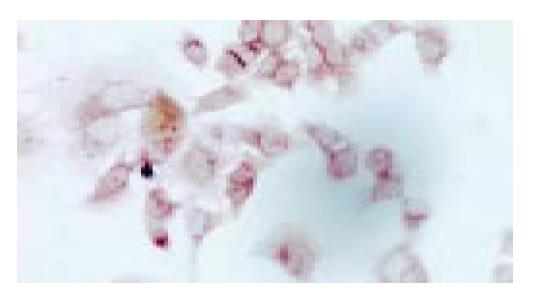
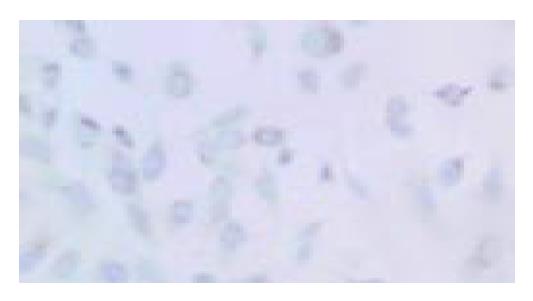
Cells crawling on the slides were stained by immunocytochemical staining. SST2 gene expressing PC-3 cells would present brown particles spreading mainly in the cytoplasm and cell membrane (Figure 1). PC-3 cells non-transfected were negative-expression (Figure 2). We proved that PC-3 cells did not express SST2. In contrast, they could express SST2 after transfected with SST2 gene. And it also proved that the transfection was effective and accorded with the requirement of our experiments.
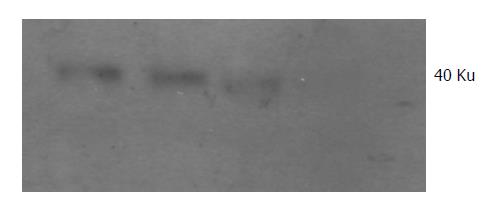
Protein of transfected PC-3 cells was extracted and the expression of SST2 was detected by Western blotting. SST2 in transfected PC-3 cells was detected (Figure 3), the Mr is about 40 000. It also proved that the transfection was effective at protein level. This was the base of our next experiments.
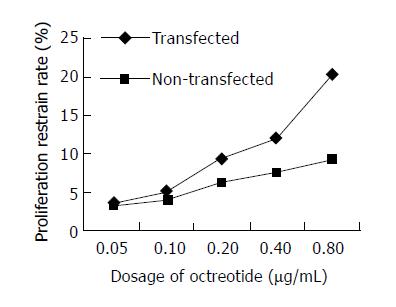
Inhibition rate was detected by MTT assay. Growth inhibition rate of transfected PC-3 cells caused by octreotide was 3.45 ± 0.91% at the dosage of 0.05 µg/mL, 5.18 ± 1.21% at the dosage of 0.10 µg/mL, 9.36 ± 1.34% at the dosage of 0.20 µg/mL, 12.03 ± 1.44% at the dosage of 0.40 µg/mL and 20.23 ± 4.21% at the dosage of 0.80 µg/mL. The inhibition rate caused by octreotide for non-transfected PC-3 cells was 3.28 ± 0.54% at the dosage of 0.05 µg/mL, 4.08 ± 0.45 % at the dosage of 0.10 µg/mL, 6.44 ± 0.66% at the dosage of 0.20 µg/mL, 7.65 ± 0.88% at the dosage of 0.40 µg/mL and 9.29 ± 1.32% at the dosage of 0.80 µg/mL. After using octreotide (0.05-0.80 µg/mL) for 48 h, we found that it caused a higher inhibition rate in transfected groups than in non-transfected groups P < 0.05) at different dosages (0.20, 0.40 and 0.80 µg/mL). It proved that the expression of SST2 could enhance octreotide’s effect on the inhibition of PC-3 cells (Figure 4).
When 0.05-0.80 µg/mL octreotide was added and incubated for 48 h, the PC-3 cells would show the typical apoptosis peak measured by flow cytometry. Moreover, the higher the drug dosage was, the higher the apoptosis rate was. Higher dosages (0.20, 0.40 and 0.80 µg/mL) of octreotide caused a greater increase of inhibition in PC-3 cells transfected groups than in non-transfected groups (P < 0.05) (Table 1).
| Dosage of octreotide (μg/mL) | Apoptosis rate of transfected PC-3 cells (%) | Apoptosis rate of non-transfected PC-3 cells (%) | P |
| 0.05 | 3.65 ± 0.66 | 2.77 ± 0.33 | > 0.05 |
| 0.10 | 4.45 ± 0.78 | 3.23 ± 0.57 | > 0.05 |
| 0.20 | 7.56 ± 1.06 | 5.76 ± 0.75 | < 0.05 |
| 0.40 | 9.25 ± 1.73 | 6.69 ± 0.80 | < 0.05 |
| 0.80 | 14.18 ± 2.71 | 7.26 ± 1.28 | < 0.05 |
At present, somatostatin has been widely used in therapy of various tumors especially endocrine tumors. In a majority of cases, favorable effect has been noted. In combined treatment with classical chemotherapeutic drugs, such as 5-fluorouracil, somatostatin has synergistic action of killing tumor cells[5]. The mechanism of somatostatin’s anti-tumor effect in vitro lies in combining with its receptor. Somatostatin receptor belongs to a protein G coupled receptor family[6]. According to the similarity of structure and difference in affinity to somatostatin, somatostatin receptors are classified into two categories: (1) having strong affinity to somatostatin, including SST2A, SST2B, SST3 and SST5; (2) having weak affinity to somatostatin, including SST1 and SST4[7,8]. It is known that somatostatin receptors are extensively distributed in human central nervous system and other tissues. The category and amount of somatostatin receptor expression is different in various tumors. Somatostatin receptor, especially SST2 is usually highly expressed in many tumor tissues. SST2 may play a role in anti-tumor effect of somatostatin (It is considered as a tumor suppressing gene)[9-11]. The mechanisms of somatostatin’s anti-tumor action are as follows: (1) direct negative effect on tumor growth; (2) inhibiting the growth of tumors by interfering with synthesis of growth factors, which are produced via paracrine and autocrine of tumor cells; (3) anti-tumor effects by inhibiting excretion of somatotropin, insulin, gastrin and other hormones or depressing insulin-like growth factor-I (IGF-I), epidermal growth factor (EGF) and other growth factors; (4) interference with DNA synthesis of tumor cells; (5) suppression of angiogenesis of tumors[12].
Substantial evidence has shown that loss or low expression of SST2 was observed in primary pancreatic cancer[9,13-15]. Our previous research results have also shown that expression of SST2 was detected in 12.5 % of primary pancreatic cancers. These results suggest that expression of SST2 was deficient in a majority of pancreatic cancer tissues, which may be the main reason why somatostatin and somatostatin analogues were nearly inefficacious in the treatment of pancreatic cancer. But it is interesting that expression of SST2 was observed in the majority of adjacent histologically noncancerous pancreas, which was beneficial for somatostatin and somatostatin analogues to suppress fast invasion and growth of tumor cells. These results also suggest that if expression of SST2 in pancreatic cancer and adjacent histologically noncancerous pancreas was reduced; somatostatin and somatostatin analogues were nearly inefficacious in the treatment of pancreatic cancer. But if expression of SST2 in both pancreatic cancer and adjacent histologically noncancerous pancreas were observed, somatostatin and somatostatin analogues were efficacious[11,1]. Therefore, different expression of SST2 in pancreatic cancer and adjacent histologically noncancerous pancreas is bound to result in different effects of treatment, which may be the main reason why reports of effects of somatostatin and somatostatin analogues treatment on pancreatic cancer are inconsistent at the present time. We believe that it is very important to make clear whether SST2 is expressed in pancreatic cancer and adjacent histologically noncancerous pancreas before somatostatin and somatostatin analogues are used in treatment of pancreatic cancer. We have demonstrated that transfecting SST2 gene into human pancreatic cancer cells which lack SST2 could markedly up-regulate SST2 expression, increase apoptosis rate of human pancreatic cancer cells and enhance somatostatin analogue octreotide to inhibit growth of human pancreatic cancer cells and induce apoptosis. These results suggest that it may be an exciting molecular biological therapy for human pancreatic cancer deficiency of SST2 gene via transfection with SST2 gene and combination with somatostatin analogue.
Edited by Zhu LH Proofread by Chen WW and Xu FM
| 1. | Guillermet J, Saint-Laurent N, Rochaix P, Cuvillier O, Levade T, Schally AV, Pradayrol L, Buscail L, Susini C, Bousquet C. Somatostatin receptor subtype 2 sensitizes human pancreatic cancer cells to death ligand-induced apoptosis. Proc Natl Acad Sci USA. 2003;100:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Moneta D, Richichi C, Aliprandi M, Dournaud P, Dutar P, Billard JM, Carlo AS, Viollet C, Hannon JP, Fehlmann D. Somatostatin receptor subtypes 2 and 4 affect seizure susceptibility and hippocampal excitatory neurotransmission in mice. Eur J Neurosci. 2002;16:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Hofland LJ, Lamberts SW. Somatostatin receptor subtype expression in human tumors. Ann Oncol. 2001;12 Suppl 2:S31-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Raderer M, Hamilton G, Kurtaran A, Valencak J, Haberl I, Hoffmann O, Kornek GV, Vorbeck F, Hejna MH, Virgolini I. Treatment of advanced pancreatic cancer with the long-acting somatostatin analogue lanreotide: in vitro and in vivo results. Br J Cancer. 1999;79:535-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Weckbecker G, Raulf F, Tolcsvai L, Bruns C. Potentiation of the anti-proliferative effects of anti-cancer drugs by octreotide in vitro and in vivo. Digestion. 1996;57 Suppl 1:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Burchett SA, Flanary P, Aston C, Jiang L, Young KH, Uetz P, Fields S, Dohlman HG. Regulation of stress response signaling by the N-terminal dishevelled/EGL-10/pleckstrin domain of Sst2, a regulator of G protein signaling in Saccharomyces cerevisiae. J Biol Chem. 2002;277:22156-22167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Prevost G, Veber N, Viollet C, Roubert V, Roubert P, Benard J, Eden P. Somatostatin-14 mainly binds the somatostatin receptor subtype 2 in human neuroblastoma tumors. Neuroendocrinology. 1996;63:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, O'Carroll AM, Patel YC, Schonbrunn A, Taylor JE. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995;16:86-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 416] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 9. | Rochaix P, Delesque N, Estève JP, Saint-Laurent N, Voight JJ, Vaysse N, Susini C, Buscail L. Gene therapy for pancreatic carcinoma: local and distant antitumor effects after somatostatin receptor sst2 gene transfer. Hum Gene Ther. 1999;10:995-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Hofland LJ, Lamberts SW. Somatostatin analogs and receptors. Diagnostic and therapeutic applications. Cancer Treat Res. 1997;89:365-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Paillard F. Somatostatin receptor gene transfer induces bystander effects. Hum Gene Ther. 1999;10:857-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Zaki M, Harrington L, McCuen R, Coy DH, Arimura A, Schubert ML. Somatostatin receptor subtype 2 mediates inhibition of gastrin and histamine secretion from human, dog, and rat antrum. Gastroenterology. 1996;111:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Froidevaux S, Hintermann E, Török M, Mäcke HR, Beglinger C, Eberle AN. Differential regulation of somatostatin receptor type 2 (sst 2) expression in AR4-2J tumor cells implanted into mice during octreotide treatment. Cancer Res. 1999;59:3652-3657. [PubMed] |
| 14. | Delesque N, Buscail L, Estève JP, Saint-Laurent N, Müller C, Weckbecker G, Bruns C, Vaysse N, Susini C. sst2 somatostatin receptor expression reverses tumorigenicity of human pancreatic cancer cells. Cancer Res. 1997;57:956-962. [PubMed] |
| 15. | Buscail L, Saint-Laurent N, Chastre E, Vaillant JC, Gespach C, Capella G, Kalthoff H, Lluis F, Vaysse N, Susini C. Loss of sst2 somatostatin receptor gene expression in human pancreatic and colorectal cancer. Cancer Res. 1996;56:1823-1827. [PubMed] |