INTRODUCTION
Inflammatory pseudotumors of the liver are rare benign lesions characterized by proliferating fibrovascular tissue mixed with inflammatory cells. They are associated with fever, pain and a mass effect, and are commonly mistaken for malignant tumors or liver abscesses. Usually the radiological or cytological examinations fail to differentiate hepatic pseudotumors from liver neoplasms. We report a patient with an inflammatory tumor of the liver who was found to have a malignant gastrointestinal stromal tumor (GIST) of the small bowel at the same time, with special emphasis on their possible etiological association and the diagnostic dilemma.
CASE REPORT
A 46-year-old housewife was admitted to our hospital in January 1998 with right upper quadrant abdominal pain and low grade fever for one week. Blood tests showed leukocytosis (16.7×109/L) and raised erythrocyte sedimentation rate (ESR) (92 mm/h). Serum alkaline phosphatase (138IU/L) was slightly increased but hepatic parenchymal enzymes were normal. The levels of serum alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) were within normal ranges. Hepatitis B and C viral serology tests were negative. Repeated blood culture did not reveal any bacterial growth.
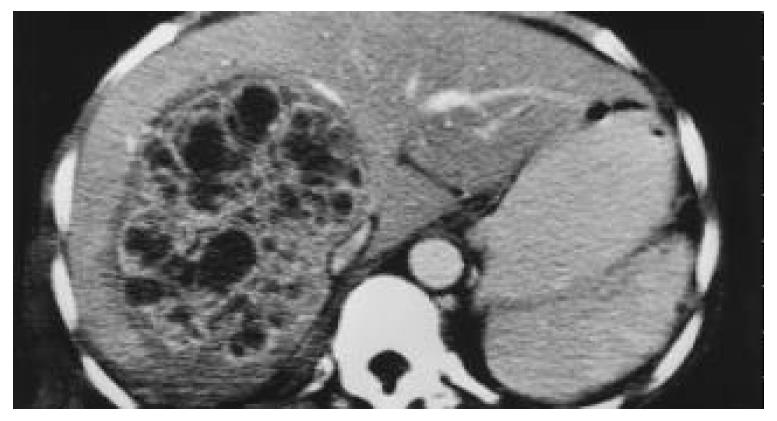
In view of the mildly deranged liver function, an abdominal ultrasonography was performed which showed a space-occupying lesion in the right hepatic lobe. No dilated intrahepatic or common bile ducts were noted. Helical computer tomography (CT) scan showed a heterogeneous lesion of 10 cm in size in the right lobe of the liver (Figure 1). The right portal vein was displaced anteriorly. Radiologist commented that it could be a large multi-septated liver abscess, but the possibility of a liver tumor could not be excluded.
Figure 1 Contrast CT scan showing heterogeneous contrast enhancement in the lesion with a multi-septated appearance.
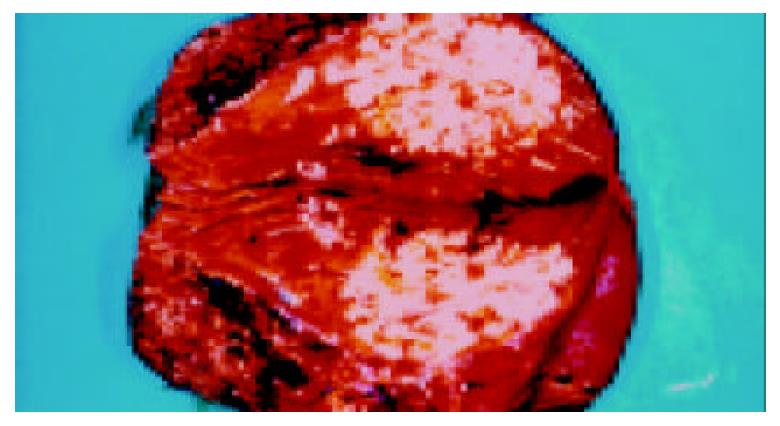
The patient was treated initially with intravenous cefotaxime and metronidazole, and the fever subsided after 1 wk of antibiotics treatment. However, due to the large size of the lesion and the worry about possible malignancy, surgical exploration was decided. Intraoperatively, in addition to the liver mass, a 10 cm soft tissue tumor was found in the ileum at 15 cm from the ileocecal valve. The tumor and a segment of the small bowel were resected and frozen section examination showed a spindle cell tumor. Trucut biopsy of the liver mass and frozen section suggested a metastastic spindle cell tumor. In view of an isolated hepatic metastasis without lymph node involvement or other evidence of metastasis, an extended right hepatectomy with a 1 cm resection margin from the tumor was performed (Figure 2).
Figure 2 Yellowish firming well-circumscribed mass in the right hepatectomy specimen.
Histopathological examination of the paraffin section of the small bowel tumor confirmed the diagnosis of a malignant GIST. Microscopic examination of the paraffin section of the liver “tumor” showed that it was composed of fascicles of spindle fibroblasts and myofibroblasts admixed with chronic inflammatory cells, which were mainly lymphocytes and plasma cells. The overall pathological features were those of an inflammatory pseudotumor of the liver.
The patient recovered well after the operation and was discharged without complication. The patient had regular follow-up in our outpatient clinic with yearly CT scan. After 4 years of follow-up, the patient was in good health and there was no evidence of tumor recurrence or further liver lesion.
DISCUSSION
Inflammatory pseudotumor of the liver is a rare clinical and pathological entity. Pack and Baker first described it in a patient after right hepatic lobectomy in 1953[1]. To date, there have been fewer than 80 cases reported in the literature[2-7]. These “tumors” were so named because of the difficulty in distinguishing them from malignant lesions preoperatively, as in the case of our patient. Histologically, these tumors are composed of densely hyalinized collagenous tissue infiltrated by a variety of cells, mainly plasma cells and the enigmatic plump spindle cells, although monocytes and lymphocytes have also been found[3]. The pathogenesis and etiology of inflammatory pseudotumors of the liver are uncertain. Some authors suggested that they might be attributed to a septic origin from aberrant inflammatory reaction to migrating microorganisms from large bowel[2]. Though bacterial culture growths, such as Escherichia coli and Klebsiella pneumoniae, have been reported in a few cases[8], no definite microorganism could be isolated from the specimens in most cases. Other authors suggested that Epstein Barr viral infection might play a role in the pathogenesis of inflammatory pseudotumors[9], but this still needs to be verified by further studies.
In the reported cases, patients with an inflammatory pseudotumor of the liver usually complained of non-specific symptoms at first presentation, such as abdominal pain, fever, weight loss and malaise. Elevated ESR, leukocytosis and mildly elevated hepatic transaminases and bilirubin were typical. Inflammatory pseudotumor has been reported in children as young as 3 mo of age and in adults up to 83 years of age[4,5], with male preponderance. Schmid reported that more patients with inflammatory pseudotumors were observed in Southeast Asians (54.7%) when compared with other Western counterparts[4]. As hepatitis B infection is common in Southeast Asia, hepatocellular carcinoma is the most common liver tumor encountered in this area. However, inflammatory pseudotumor should be included in the differential diagnosis of a hepatic mass, especially in those patients with normal hepatitis B screening and AFP level.
The recommended therapeutic approach for inflammatory pseudotumors of the liver is still controversial, though surgical resection is generally considered as an overtreatment[8,10]. Spontaneous regression of the lesion was reported in some cases, and cases that regressed with the use of antibiotics[11] or nonsteroidal anti-inflammatory drugs[12] have also been reported. When the inflammatory pseudotumors caused major complications, such as biliary obstruction[13] and portal hypertension[14], the patients might need surgical resection or even liver transplantation[15]. Although most authors believed that inflammatory pseudotumors had a benign behavior, there have been some reports of invasive course and mortality[2].
To our knowledge, this is the first patient reported to have an inflammatory pseudotumor in association with a small bowel
GIST. The exact etiological link between the two is uncertain. However, the presence of an ileal GIST could potentially enhance entry of enteric bacteria into the portal circulation, which could then result in an inflammatory pseudotumor if the hypothesis of an infective etiology is true. In our patient, frozen section examination of the hepatic mass suggested that it was a metastasis from the malignant small bowel GIST, but we proceeded with hepatic resection in view of the absence of other obvious extrahepatic metastasis. In the past, patients with hepatic metastases from malignant GIST or leiomyosarcoma were considered to have poor prognosis even after surgical resection[16,17]. Hence, hepatic resection was usually not offered to patients with liver metastasis from gastrointestinal leiomyosarcoma. However, recently some authors advocated that aggressive surgical resection for liver metastasis might provide survival benefit to the patients[18,19]. In a recent report from a single institution, a median survival of 40 mo and a 5-year survival rate of 33% were observed after hepatic resection for hepatic metastases from leiomyosarcoma[17]. We adopted a similar approach and performed a major hepatectomy. The final pathology of the liver lesion turned out to be an inflammatory pseudotumor, and the patient had survived without any disease recurrence for four years by the time of writing the manuscript. Our case illustrated the difficulty in differentiating an inflammatory pseudotumor from a malignancy not only in the preoperative imaging but also even by frozen section examination. The histological appearance of an inflammatory pseudotumor and a GIST is particularly difficult to differentiate. The surgeons should be aware of their possible association as illustrated in this case. A favorable long-term outcome may be expected with resection of the GIST together with hepatic resection for the inflammatory pseudotumor in the liver.