Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1826
Revised: December 9, 2003
Accepted: December 16, 2003
Published online: June 15, 2004
AIM: To investigate the relationships between polymorphisms of interleukin-1B (IL-1B) promoter region -511C/T and interleukin-1 receptor antagonist gene (IL-1RN) and susceptibility to chronic hepatitis B in Chinese population.
METHODS: Genomic DNA was extracted from the peripheral blood of 190 patients with chronic hepatitis B and 249 normal controls and then subjected to polymerase chain reaction (PCR) amplification. The PCR products were digested by restriction endonuclease AvaI. The products of digestion were subjected to 20 g/L gel electrophoresis and ethidium bromide staining.
RESULTS: The frequencies of IL-1B (-511) genotypes CC, CT and TT in patients with chronic hepatitis B were 23.7%, 49.5% and 26.8%, while 26.1%, 47.4% and 26.5% respectively in controls. The results showed that there was no significant difference in the frequencies of alleles or genotypes in IL-1B between patients with chronic hepatitis B and controls. The distributions of IL-1B (-511) genotype CC were significantly different between the two subgroups (HBV-DNA ≤ 1×103 copies/mL as subgroup I, HBV-DNA > 1×103 copies/mL as subgroup II) of chronic hepatitis B (P = 0.029). Only four of the five kinds of polymorphism (1/1, 1/2, 2/2 and 1/4) were found in this study. The frequencies of IL-1RN genotypes 1/1, 1/2, 2/2 and 1/4 were 88.9%, 9.0%, 0.5% and 1.6% in patients with chronic hepatitis B respectively, while were 81.1%, 16.9%, 0.4% and 1.6% respectively in controls. The frequencies of genotype1/2 and IL-1RN allele 2 in patients with chronic hepatitis B were lower than those in controls (P = 0.016 and P = 0.029, respectively).
CONCLUSION: There is an association between polymorphisms of the promoter region -511C/T of IL-1B and IL-1RN intron 2 and chronic hepatitis B virus infection. Subjects with IL-1RN allele 2 may be resistant to HBV infection, and IL-1B (-511) genotype CC is closely related with HBV-DNA replication, which gives some new clues to the study of pathogenesis of chronic hepatitis B.
- Citation: Zhang PA, Li Y, Xu P, Wu JM. Polymorphisms of interleukin-1B and interleukin-1 receptor antagonist genes in patients with chronic hepatitis B. World J Gastroenterol 2004; 10(12): 1826-1829
- URL: https://www.wjgnet.com/1007-9327/full/v10/i12/1826.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i12.1826
Hepatitis B virus (HBV) infection is one of the most important chronic viral diseases in the world. An estimated 400 million people worldwide are carriers of HBV, and approximately 250 000 deaths occur each year as a consequence of fulminant hepatic failure, cirrhosis, and hepatocellular carcinoma[1]. When HBV is acquired in adulthood, the majority of infections are cleared, with chronic infection occurring in 5% to 10% of cases. However, the dynamic interaction of the host inflammatory response with HBV, and the subsequent impact of this interaction on the clinical outcome of HBV infection, are not yet fully understood, nor are the underlying mechanisms for persistence of the virus.
Cytokines play an important role in defense against viral infection, indirectly through determination of the predominant pattern of the host response, and directly through inhibition of viral replication[2]. Interleukin(IL)-1 is one of the most proinflammatory agents, and has a central role in inflammation and destruction[3]. The most important members of the IL-1 family are the IL-1α, IL-1β and IL-1 receptor antagonists (ra). IL-1ra is an IL-1 natural competitive inhibitor, acting by occupancy of cell surface receptor without triggering signal transduction[4]. IL-1ra plays a role as an important regulator of inflammation and is currently evaluated in clinical trials. Genes encoding IL-1 are located on the 430 kb region of chromosome 2q13-21, consisting of three homologous genes: IL-1A, IL-1B and IL-1ra (IL-1RN)[5]. Biallelic polymorphisms at positions IL-1A -889, IL-1B -511, and +3 953 have been described, all representing a C/T single nucleotide polymorphism (SNP). IL-1RN contains an 86 bp variable number tandem repeat (VNTR) polymorphism in intron 2[6]. These polymorphisms are located within the regulatory regions of the genes and have potential functional importance by modulating IL-1 protein production, and are related with the development of some diseases[7].
This research was to discuss the relationship between polymorphisms of IL-1 gene and susceptibility to HBV in the Chinese population, and tried to reveal the correlation between the genotype and phenotype distributions, in order to provide a certain scientific basis for prevention and treatment of chronic hepatitis B.
A total of 190 patients with chronic hepatitis B (130 males, 60 females) aged 11-68 years were recruited in Remin Hospital of Wuhan University. The diagnosis of all the patients was confirmed according to the criteria for chronic hepatitis B, and the patients did not have other viral hepatitis. Two hundred and forty-nine control subjects (150 males, 99 females) aged 18-82 years were selected in Wuhan area (HBsAg negative, anti-HBe negative, and anti-HBc negative), and liver, renal, endocrine and cardiovascular disorders were excluded.
Two microliter peripheral venous blood was collected in an EDTA tube. Genomic DNA was extracted from peripheral blood leukocytes and frozen at -20 °C. Each PCR was carried out in 25 μL reaction mixture containing 100-200 ng genomic DNA, 100 μmol/L dNTP, 25 mmol/L MgCl2, 20 pmol/L primers and 1U TaqDNA polymerase (Promega).
SNP at position -511 in the promoter region (-702-398) of IL-1B was analyzed by the PCR-restriction fragment length polymorphism (RFLP) method[8]. A 304 bp PCR fragment of the IL-1B promoter region was amplified using the following primers: 5’-TGGCATTGATCTGGTTCATC-3’ and 5’-GTTTAGGATCTTCCCACTT-3’. PCR conditions were as follows: denaturation at 95 °C for 5 min, then 35 cycles at 94 °C for 30 s, at 55 °C for 30 s, at 72 °C for 1 min, and finial extention at 72 °C for 5 min. twenty μL of PCR products was digested with 5 U of AvaI (TaKaRa, shiga, Japan) at 37 °C for 3 h and run on a 20 g/L agarose gel stained with ethidium bromide.
IL-1RN intron 2 contained a VNTR of an 86 bp length of DNA. Oligonucleotides 5’-CTCAGCAACACTCCTAT-3’ and 5’-TCCTGGTCTGCAGGTAA-3’ flanking this region were used as primers[8]. Amplification conditions were same as the above. The PCR products were analyzed by electrophoresis on a 20 g/L agarose gel stained with ethidium bromide.
Serum HBV-DNA level in patients with chronic hepatitis B was detected with the real-time fluorescent quantitative PCR method (reagents supplied by Shanghai Fosun Co. Ltd.) using a Lightcycler PCR system. Results were considered abnormal when HBV-DNA > 1×103copies/mL.
IL-1B (-511) and IL-1RN (intron 2) allelic and genotype frequencies were calculated in patients with chronic hepatitis B and control subjects. Comparison of allelic and genotypes between groups, and association of IL-1B and IL-1RN polymorphisms with HBV-DNA replication were examined for statistical significance with chi-square test. Analysis was completed with SPSS 9.0, and P < 0.05 was considered statistically significant.
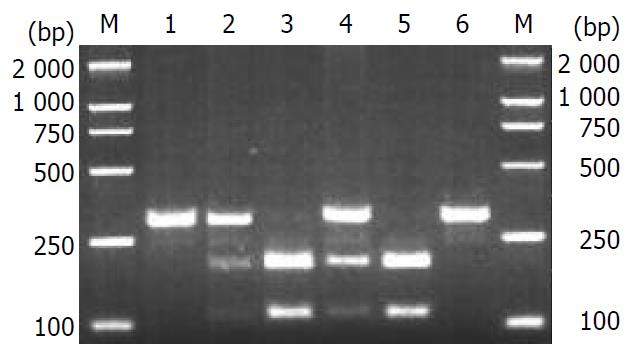
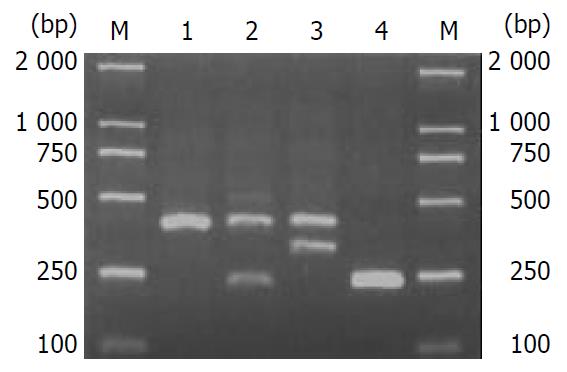
C/T transition polymorphism of IL-1B (-511) was analyzed by PCR-RFLP. AvaI digestion of the PCR products of IL-1B resulted in three genotypes, consisting of TT (intact, 304 bp), CT (three fragments of 304, 190 and 114 bp) and CC (two fragments of 190 and 114 bp), (Figure 1). The intron 2 of IL-1RN polymorphism contained VNTR of 86 bp. There were five alleles in humans, including allele 1 (four repeats, 410 bp), allele 2 (two repeats, 240 bp), allele 3 (five repeats, 500 bp), allele 4 (three repeats, 325 bp) and allele 5 (six repeats, 595 bp). Only four of the five kinds of polymorphism of IL-1RN (1/1, 1/2, 2/2, and 1/4) were found in this study (Figure 2).
The genotype and allele frequencies of IL-1B and IL-1RN in patients with chronic hepatitis B and control subjects were determined, and explored with 2×2 χ2 test under the Hardy-Weinberg law and shown to represent all population (Table 1, Table 2). The frequencies of IL-1B genotypes CC, CT and TT in patients with chronic hepatitis B were 23.7% (45/190), 49.5% (94/190) and 26.8% (51/190), while those in controls were 26.1% (65/249), 47.4% (118/249), and 26.5% (66/249) respectively. The results showed that there was no significant difference in the frequencies of alleles (χ2 = 0.16, P = 0.69) or genotypes (χ2 = 0.35, P = 0.84) in IL-1B between patients with chronic hepatitis B and control subjects.
| Group | n | Frequency of genotypes (%) | Frequency of alleles (%) | |||
| CC | CT | TT | C | T | ||
| Patients | 190 | 45 (23.7) | 94 (49.5) | 51 (26.8) | 184 (48.4) | 196 (51.6) |
| Controls | 249 | 65 (26.1) | 118 (47.4) | 66 (26.5) | 248 (49.8) | 250 (50.2) |
Only four of the five kinds of polymorphism of IL-1RN (1/1, 1/2, 2/2 and 1/4) were found in this study. The frequencies of 1/1, 1/2, 2/2 and 1/4 were 88.9%, 9.0%, 0.5% and 1.6% in chronic hepatitis B patients, while those in controls were 81.1%, 16.9%, 0.4% and 1.6% respectively. IL-1 RN allele 1 was detected in 94.2% of chronic hepatitis B patients and 90.4% of controls, while IL-1RN allele 2 was detected in 5.0% of the patients and 8.8% of controls, The frequencies of genotype 1/2 and IL-1RN allele 2 in patients with chronic hepatitis B were lower than those in controls (χ2 = 5.81, P = 0.016 and χ2 = 4.76, P = 0.029 respectively).
For further analysis of the relationship between IL-1B, IL-1RN polymorphisms and HBV-DNA replication in patients with chronic hepatitis B, the patients were divided into two subgroups (HBV-DNA ≤ 1×103 copies/mL as subgroup I, HBV-DNA > 1×103 copies/mL as subgroup II). As shown in Table 3, the distributions of IL-1B (-511) genotype CC were of significant difference between the two subgroups (χ2 = 4.77, P = 0.029).
HBV infection is a major global health problem with an estimated 300 million people chronically infected worldwide[9,10]. Individuals with an inadequate primary immune response to HBV are at increased risk of developing chronic hepatitis B. Age is the strongest host feature associated with chronic infection with 90% infants and 5%-10% of adults developing chronic hepatitis B after exposure. In addition, people with the same age, sex and ethnical group were exposed to the same HBV strain, which could cause a broad spectrum ranging from no infection to different clinical outcomes[11]. These data suggest that host genetic factors are responsible for the clinical outcomes of HBV infection. Clearance of HBV requires a coordinated innate and adaptive humoral and cell-mediated immune response. Cytokines are soluble polypeptide molecules that mediate cell-to-cell communication and regulate the intensity and duration of the immune response. Previous studies have shown that the maximal capacity of cytokine production varies among individuals and correlates with SNP in the promoter region of various cytokine genes[12,13]. Furthermore, cytokine gene polymorphisms were associated with liver disease severity in patients with viral hepatitis[14]. In the present study we compared the distributions of IL-1B (-511) and IL-1RN intron 2 VNTR polymorphisms between patients with chronic B and control subjects.
IL-1B is one of the IL-1 gene family members, and located in the proximal region of chromosome 2q13-21. Different polymorphisms have been described in IL-1B, and at least two of them could influence the protein production. One is located in the promoter region at position -511, and the other in exon 5. Pociot et al[15] reported that IL-1B polymorphisms were correlated with IL-1β expression. IL-1B allele T carrier had higher productions of IL-1β than IL-1B allele C carrier. In the study, genotype distributions and allelic frequencies for IL-1B (-511) promoter polymorphisms in patients with chronic hepatitis B and control subjects were not statistically different. Further analysis of the relationship between IL-1B polymorphism and HBV-DNA replication in patients with chronic hepatitis B showed that IL-1B (-511) genotype CC was associated with HBV-DNA replication.
IL-1ra is a naturally occurring anti-inflammatory protein, competitively blocks the binding of IL-1α and IL-1β type I and type II IL-1 receptors, but exerts no agonist activity, despite sharing 30% amino acid sequence homology with IL-1β, and 19% with IL-1α. Il-1ra has been shown to inhibit the effects of IL-1 both in vitro and in vivo[16]. Increasing evidence showed that IL-1RN polymorphisms were related with susceptibility to individual diseases, including psoriasis, systemic lupus erthematosus, and inflammatory bowel disease[4]. By the study of IL-1RN intron 2 polymorphisms, our results suggested that the distributions of IL-1RN 1/2 genotype and IL-1RN allele 2 in patients with chronic hepatitis B were significantly lower than those in control subjects. A possible explanation of this result could be provided by the fact that carriage of Il-1RN allele 2 of each of these polymorphisms was related with high production of IL-1β[17], which may augment the production of other cytokines, such as IL-2, IL-6 and TNF-α, and trigger the complex immuological processes to eliminate the virus and its complex.
In summary, the findings of this study and others may provide further evidence that genetic factors are important in the pathogenesis of HBV infection. Our results suggest that the carriage of IL-1RN allele 2 may play a protective role in the development of HBV infection and IL-1B CC genotype may be associated with HBV-DNA replication. However, the real roles of IL-1 polymorphisms in the pathogenesis of developing chronic hepatitis B should be further investigated by large population-based studies.
Edited by Zhang JZ and Wang XL Proofread by Xu FM
| 1. | Perrillo RP. How will we use the new antiviral agents for hepatitis B. Curr Gastroenterol Rep. 2002;4:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Koziel MJ. Cytokines in viral hepatitis. Semin Liver Dis. 1999;19:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Hutyrová B, Pantelidis P, Drábek J, Zůrková M, Kolek V, Lenhart K, Welsh KI, Du Bois RM, Petrek M. Interleukin-1 gene cluster polymorphisms in sarcoidosis and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;165:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Ma P, Chen D, Pan J, Du B. Genomic polymorphism within interleukin-1 family cytokines influences the outcome of septic patients. Crit Care Med. 2002;30:1046-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Nemetz A, Nosti-Escanilla MP, Molnár T, Köpe A, Kovács A, Fehér J, Tulassay Z, Nagy F, García-González MA, Peña AS. IL1B gene polymorphisms influence the course and severity of inflammatory bowel disease. Immunogenetics. 1999;49:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Garcia-Gonzalez MA, Lanas A, Santolaria S, Crusius JB, Serrano MT, Peña AS. The polymorphic IL-1B and IL-1RN genes in the aetiopathogenesis of peptic ulcer. Clin Exp Immunol. 2001;125:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Moos V, Rudwaleit M, Herzog V, Höhlig K, Sieper J, Müller B. Association of genotypes affecting the expression of interleukin-1beta or interleukin-1 receptor antagonist with osteoarthritis. Arthritis Rheum. 2000;43:2417-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Kanemoto K, Kawasaki J, Miyamoto T, Obayashi H, Nishimura M. Interleukin (IL)1β, IL-1α, and IL-1 receptor antagonist gene polymorphisms in patients with temporal lobe epilepsy. Ann Neurol. 2000;47:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Wang FS. Current status and prospects of studies on human genetic alleles associated with hepatitis B virus infection. World J Gastroenterol. 2003;9:641-644. [PubMed] |
| 10. | Cacciola I, Cerenzia G, Pollicino T, Squadrito G, Castellaneta S, Zanetti AR, Mieli-Vergani G, Raimondo G. Genomic heterogeneity of hepatitis B virus (HBV) and outcome of perinatal HBV infection. J Hepatol. 2002;36:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Rapicetta M, Ferrari C, Levrero M. Viral determinants and host immune responses in the pathogenesis of HBV infection. J Med Virol. 2002;67:454-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Giedraitis V, He B, Huang WX, Hillert J. Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol. 2001;112:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 354] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Ben-Ari Z, Mor E, Papo O, Kfir B, Sulkes J, Tambur AR, Tur-Kaspa R, Klein T. Cytokine gene polymorphisms in patients infected with hepatitis B virus. Am J Gastroenterol. 2003;98:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 180] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Miyazoe S, Hamasaki K, Nakata K, Kajiya Y, Kitajima K, Nakao K, Daikoku M, Yatsuhashi H, Koga M, Yano M. Influence of interleukin-10 gene promoter polymorphisms on disease progression in patients chronically infected with hepatitis B virus. Am J Gastroenterol. 2002;97:2086-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Pociot F, Mølvig J, Wogensen L, Worsaae H, Nerup J. A TaqI polymorphism in the human interleukin-1 beta (IL-1 beta) gene correlates with IL-1 beta secretion in vitro. Eur J Clin Invest. 1992;22:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 694] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 16. | Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J Clin Invest. 1997;99:2930-2940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 220] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Santtila S, Savinainen K, Hurme M. Presence of the IL-1RA allele 2 (IL1RN*2) is associated with enhanced IL-1beta production in vitro. Scand J Immunol. 1998;47:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 408] [Article Influence: 15.1] [Reference Citation Analysis (0)] |