Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1822
Revised: January 8, 2004
Accepted: January 15, 2004
Published online: June 15, 2004
AIM: To investigate the apoptosis in primary gastric cancer cells induced by genistein, and the relationship between this apoptosis and expression of bcl-2 and bax.
METHODS: MTT assay was used to determine the cell growth inhibitory rate in vitro. Transmission electron microscope and TUNEL staining were used to quantitatively and qualitatively detect the apoptosis of primary gastric cancer cells before and after genistein treatment. Immunohistochemical staining and RT-PCR were used to detect the expression of apoptosis-associated genes bcl-2 and bax.
RESULTS: Genistein inhibited the growth of primary gastric cancer cells in dose-and time-dependent manner. Genistein induced primary gastric cancer cells to undergo apoptosis with typically apoptotic characteristics. TUNEL assay showed that after the treatment of primary gastric cancer cells with genistein for 24 to 96 h, the apoptotic rates of primary gastric cancer cells increased time-dependently. Immunohistochemical staining showed that after the treatment of primary gastric cancer cells with genistein for 24 to 96 h, the positivity rates of Bcl-2 proteins were apparently reduced with time and the positivity rates of Bax proteins were apparently increased with time. After exposed to genistein at 20 μmol/L for 24, 48, 72 and 96 respectively, the density of bcl-2 mRNA decreased progressively and the density of bax mRNA increased progressively with elongation of time.
CONCLUSION: Genistein is able to induce the apoptosis in primary gastric cancer cells. This apoptosis may be mediated by down-regulating the apoptosis- associated bcl-2 gene and up-regulating the expression of apoptosis-associated bax gene.
- Citation: Zhou HB, Chen JJ, Wang WX, Cai JT, Du Q. Apoptosis of human primary gastric carcinoma cells induced by genistein. World J Gastroenterol 2004; 10(12): 1822-1825
- URL: https://www.wjgnet.com/1007-9327/full/v10/i12/1822.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i12.1822
Genistein is a planar molecule with an aromatic A-ring, has a second oxygen atom 11.5 Å from the one in the A ring, a molecular weight similar to those of the steroidal estrogens. It has estrogenic properties in receptor binding assays[1,2], cell culture[3,4], and uterine weight assays[5-7]. Genistein inhibits topoisomerase II[8], platelet-activating factor- and epidermal growth factor-induced expression of c-fos[9], diacylglycerol synthesis[10], and tyrosine kinases[11]. It also inhibits microsomal lipid peroxidation[12] and angiogenesis[13]. Genistein exhibits antioxidant properties[14-16] and was reported to induce differentiation of numerous cell types[17-19]. Moreover, a recent report shows that genistein is a potent cancer chemopreventive agent[20-22]. The anti-tumor activity of genistein might be related to induce the apoptosis of tumor cells but the precise mechanism of antitumor activity is not well understood.
The Bcl-2 family plays a crucial role in the control of apoptosis. The family includes a number of proteins which have homologous amino acid sequences, including anti-apoptotic members such as Bcl-2 and Bcl-xL, as well as pro-apoptotic members like Bax and Bad[23-26]. Overexpression of Bax has the effect of promoting cell death[27-31]. Conversely, Overexpression of antiapoptotic proteins such as Bcl-2 will repress the function of Bax[32-36]. Thus, the ratio of Bcl-2/Bax appears to be a critical determinant of cell apoptosis[37].
In this study, MTT assay was used to determine the cell growth inhibitory rate. Transmission electron microscope and TUNEL staining method were used to quantitatively and qualitatively detect the apoptosis status of primary gastric cancer cells before and after the genistein treatment. Immunohistochemical staining and RT-PCR were used to detect the expression of apoptosis-associated genes bcl-2 and bax.
Genistein and MTT were obtained from Sigma Chemical Co, Ltd. In situ cell detection kit, anti-Bcl-2 monoclonal antibody and anti-Bax monoclonal antibody were purchased from Beijing Zhongshan Biotechnology Co, Ltd. Stock solution of genistein was made in dimethylsulfoxide (DMSO) at a concentration of 40 µmol/ L. Working dilutions were directly made in the cell culture medium.
Cell culture Fresh sample from a patient with gastric cancer was obtained in operating room. A single-cell suspension of tumor cells with the concentration of 5×105/mL was prepared for seeding. Primary gastric cancer cells were purified after culture.
MTT assay Cells 1×105 /well in a 96-well plate after incubation for 24 h were treated with different concentrations of genistein (5, 10, 20, 40 µmol/ L) for 24, 48 and 72 h respectively. A 10 µL of 5 g/L of MTT was added to the medium triplicate at each dose and incubated for 4 h at 37 °C. Culture media were discarded followed by addition of 0.2 mL DMSO and vibration for 10 min. The absorbance (A) was measured at 570 nm using a microplate reader. The cell growth inhibitory rate was calculated as follows: {(A of control group -A of experimental group)/(A of control group- A of blank group)} ×100%.
Transmission electron microscopy Cells treated with 20 µmol/L genistein were harvested after incubation for 24 h. Subsequently the cells were fixed in 40 g/L glutaral and immersed with Epon 821, imbedded for 72 h at 60 °C. After that the cells were prepared into ultrathin section (60 nm) and stained with uranyl acetate and lead citrate. Cell morphology was observed by transmission electron microscopy.
TUNEL assay Apoptosis of primary gastric cancer cells was evaluated by using an in situ cell detection kit. The cells were treated in the presence or absence of 20 µmol/L genistein for 24 to 96 h and fixed in ice-cold 800 mL/L ethanol for up to 24 h, treated with proteinase K and then 3 mL/L H2O2, and labeled with fluorescein dUTP in a humid box for 1 h at 37 °C. Cells were then combined with POD-horseradish peroxidase, colored with DAB and counterstained with methyl green. Controls received the same treatment except the labeling of omission of fluorescein dUTP. Cells were visualized under light microscope. The apoptotic index (AI) was calculated as follows: AI = (Number of apoptotic cells/Total number)×100%.
Immunohistochemical staining Immunohistochemical staining was done by an avidinbiotin technique. Primary gastric cancer cells treated in the presence or absence of 20 µmol/L genistein for 24 to 96 h were grown on six-well glass plates and were fixed by acetone. After washed in PBS, the cells were incubated in 3 mL/L H2O2 solution at room temperature for 5 min. The cells were then incubated with anti-Bcl-2 or anti-Bax monoclonal antibody at a 1:300 dilution at 4 °C overnight. After wash of cells in PBS, the second antibody, biotinylated antirat IgG was added and the cells were incubated at room temperature for 1 h. After wash of cells in PBS, ABC compound was added and the cells were then incubated at room temperature for 10 min. DAB was used as the chromagen. After 10 min, the brown color signifying the presence of antigen bound to antibodies was detected by light microscopy and photographed at ×200. Controls were treated the same as the experimental group except the incubation of the primary antibody instead of second antibody. The positive rate (PR) was calculated as follows: PR = (Number of positive cells/Total number)×100%.
RT-PCR The primary gastric cancer cells were treated in the presence or absence of 20 µmol/L genistein for 24 to 96 h and total RNA was extracted. The concentration of RNA was determined by absorption at 260 nm. The primers for Bcl-2, Bax and β-actin were as follows: β-actin (500 bp): 5’ GTGGG GCGCCCCAGGCACCA 3’, 5’ CTCCTTAATGTCACG CACGATTTC 3’; bcl-2 (716 bp): 5’ GGAAATATGGCGC ACGCT 3’, 5’ TCACTTGTGGCCCAGAT 3’; bax (508 bp): 5’ CCAGCTCTGAGCAGATCAT 3’, 5’ TATCAGCCCA TCTTCTTCC 3’. Polymerase chain reactions were performed in a 25 μL reaction volume. PCR for Bcl-2 and β-actin was run in the following procedures: at 94 °C for 7 min, 1 circle; at 94 °C for 1 min, at 72 °C for 1 min, 30 cycles; at 72 °C for 7 min, 1 circle. PCR for Bax was run in the following procedure: 94 °C for 1 min, 60 °C for 45 s, 72 °C for 45 s, 35 cycles. Ten μL PCR product was placed onto 15 g/L agarose gel and observed by EB staining using Gel-Pro analyzer.
Data were analyzed by the paired two-tailed Student’s t test, and significance was considered when P < 0.05.
Primary gastric cancer cells were exposed to increasing concentrations (5 µmol/L to 40 µmol/L) of genistein for 24 to 72 h, respectively. Primary gastric cancer cells showed death in a dose- and time-dependent manner. The data are summarized in Table 1.
| 24 h | 48 h | 72 h | |
| Control | 0.400 ± 0.008 | 0.406 ± 0.007 | 0.404 ± 0.008 |
| 5 μmol/L genistein | 0.361 ± 0.002a | 0.334 ± 0.012b | 0.305 ± 0.004b |
| 10 μmol/L genistein | 0.325 ± 0.004d | 0.313 ± 0.003b | 0.248 ± 0.004d |
| 20 μmol/L genistein | 0.308 ± 0.003d | 0.249 ± 0.002d | 0.206 ± 0.003d |
| 40 μmol/L genistein | 0.265 ± 0.004d | 0.215 ± 0.004d | 0.159 ± 0.002d |
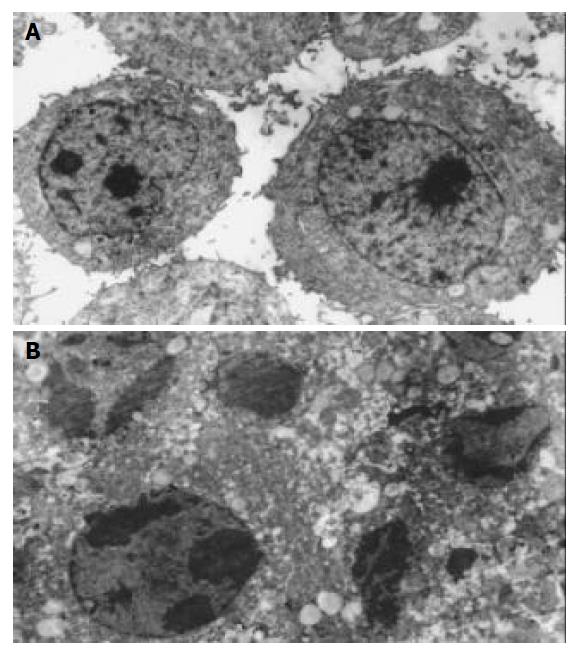
After treatment of primary gastric cancer cells with genistein (20 µmol/L) for 24 h, some cells presented apoptotic characteristics including chromatin condensation, appearance of chromatin crescent, nuclear fragmentation that could be seen by transmission electron microscope (Figure 1).
Apoptotic cells were determined by TUNEL assay according to the manufacture’s instructions. Positive staining located in the nucleus. After treatment with genistein (20 µmol/L) for 24 to 96 h, Apoptotic index of the cells was apparently increased with the increase of treatment time (P < 0.05) (Table 2).
| Control | 24 h | 48 h | 72 h | 96 h | |
| AI (%) | 1.25 ± 0.30 | 4.97 ± 0.80 | 18.44 ± 1.92 | 35.18 ± 0.35 | 43.93 ± 1.11 |
| t | -6.13 | -13.82 | -180.16 | -52.80 | |
| P | P < 0.05 | P < 0.05 | P < 0.05 | P < 0.05 |
Positive staining located in the cytoplasm. After treatment with genistein (20 µmol/L) for 24 to 96 h, Positivity rate of the cells of Bcl-2 proteins was apparently reduced with the increase of treatment periods (P < 0.05) (Table 3). This suggested that genistein could down-regulate the expression of Bcl-2.
| Control | 24 h | 48 h | 72 h | 96 h | |
| PR(%) | 36.34 ± 0.72 | 21.62 ± 0.08 | 10.60 ± 049 | 7.21 ± 0.45 | 4.54 ± 0.36 |
| t | 31.93 | 44.67 | 73.80 | 59.32 | |
| P | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 |
Positive staining located in the cytoplasm. After treatment with genistein (20 µmol/L) for 24 to 96 h, PRs of Bax proteins were apparently increased with increase of treated time (P < 0.05) (Table 4). This suggested that genistein could up-regulate the expression of Bax.
| Control | 24 h | 48 h | 72 h | 96 h | |
| PR(%) | 10.73 ± 0.57 | 20.63 ± 0.86 | 34.3 ± 0.81 | 45.96 ± 0.42 | 58.61 ± 1.46 |
| t | -57.49 | -41.23 | -69.53 | -51.31 | |
| P | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
After exposed to 20 µmol/L genistein for 24, 48, 72 and 96 h respectively, the density of bcl-2 mRNA decreased progressively and the density of bax mRNA increased progressively with elongation of time by RT-PCR. It suggested that genistein could down-regulate the expression of Bcl-2 and up-regulate the expression of Bax.
Populations who consume a diet high in soy are reported to have a lower incidence of breast and prostate cancer. The activity of the soy-derived phytoestrogen, genistein, is thought to account for a portion of the chemopreventative properties of soy; these include an inhibitory effect on tyrosine kinases[11], DNA topoisomerases I and II[8], and ribosomal S6 kinase, antiestrogenicity, antioxidant activity, anti-angiogenesis activity[12], suppression of cell proliferation, induction of differentiation[17-19], and modulation of apoptosis[20-22].
In this study, we found that primary gastric cancer cells showed death in a dose- and time-dependent manner in MTT assay. This suggested genistein was able to restrain the growth of primary gastric cancer cells. It was found that genistein was able to induce the apoptosis of primary gastric cancer cells by transmission electron microscope assay and TUNEL assay. After treatment with 20 µmol/L genistein for 24 to 96 h, PRs of Bcl-2 proteins were apparently reduced and PRs of Bax proteins were apparently increased with increase of treated time by Immunohistochemical staining. After exposed to 20 µmol/L genistein for 24, 48, 72 and 96 h respectively, the density of bcl-2 mRNA decreased progressively and the density of bax mRNA increased progressively with elongation of time by RT-PCR.
Genistein could reduce Bcl-2 expression and enhance bax expression. The ratio of Bcl-2/Bax was decreased when primary gastric cancer cells were treated with genistein, which could trigger the apoptosis of primary gastric cancer cells.
The present study demonstrated that genistein was able to induce the apoptosis in primary gastric cancer. This apoptosis may be mediated by down-regulating the expression of apoptosis-associated gene bcl-2 and up-regulating the expression of apoptosis-associated gene bax. Genistein may be used as a potential chemotherapeutic drug in the anti-gastric carcinoma chemotherapy.
Edited by Zhu LH and Chen WW Proofread by Xu FM
| 1. | Shutt DA, Cox RI. Steroid and phyto-oestrogen binding to sheep uterine receptors in vitro. J Endocrinol. 1972;52:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 258] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Mathieson RA, Kitts WD. Binding of phyto-oestrogen and oestradiol-17 beta by cytoplasmic receptors in the pituitary gland and hypothalamus of the ewe. J Endocrinol. 1980;85:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Martin PM, Horwitz KB, Ryan DS, McGuire WL. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology. 1978;103:1860-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 385] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Makela S, Davis VL, Tally WC, Korkman J, Salo L, Vihko R, Santti R, Korach KS. Dietary estrogens act through estrogen receptor-mediated processes and show no antiestrogenicity in cultured breast cancer cells. Environ Health Perspect. 1994;102:572-578. |
| 5. | Thigpen JE, Haseman JK, Saunders H, Locklear J, Caviness G, Grant M, Forsythe D. Dietary factors affecting uterine weights of immature CD-1 mice used in uterotrophic bioassays. Cancer Detect Prev. 2002;26:381-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Folman Y, Pope GS. The interaction in the immature mouse of potent oestrogens with coumestrol, genistein and other utero-vaginotrophic compounds of low potency. J Endocrinol. 1966;34:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 87] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Polkowski K, Mazurek AP. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol Pharm. 2000;57:135-155. [PubMed] |
| 8. | Okura A, Arakawa H, Oka H, Yoshinari T, Monden Y. Effect of genistein on topoisomerase activity and on the growth of [Val 12]Ha-ras-transformed NIH 3T3 cells. Biochem Biophys Res Commun. 1988;157:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 293] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Tripathi YB, Lim RW, Fernandez-Gallardo S, Kandala JC, Guntaka RV, Shukla SD. Involvement of tyrosine kinase and protein kinase C in platelet-activating-factor-induced c-fos gene expression in A-431 cells. Biochem J. 1992;286:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592-5595. [PubMed] |
| 11. | Dean NM, Kanemitsu M, Boynton AL. Effects of the tyrosine-kinase inhibitor genistein on DNA synthesis and phospholipid-derived second messenger generation in mouse 10T1/2 fibroblasts and rat liver T51B cells. Biochem Biophys Res Commun. 1989;165:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Jha HC, von Recklinghausen G, Zilliken F. Inhibition of in vitro microsomal lipid peroxidation by isoflavonoids. Biochem Pharmacol. 1985;34:1367-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 90] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, Schweigerer L. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci USA. 1993;90:2690-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 486] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 14. | Mitchell JH, Gardner PT, McPhail DB, Morrice PC, Collins AR, Duthie GG. Antioxidant efficacy of phytoestrogens in chemical and biological model systems. Arch Biochem Biophys. 1998;360:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 230] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Tikkanen MJ, Adlercreutz H. Dietary soy-derived isoflavone phytoestrogens. Could they have a role in coronary heart disease prevention. Biochem Pharmacol. 2000;60:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 126] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Wei H, Bowen R, Cai Q, Barnes S, Wang Y. Antioxidant and antipromotional effects of the soybean isoflavone genistein. Proc Soc Exp Biol Med. 1995;208:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 307] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Watanabe T, Kondo K, Oishi M. Induction of in vitro differentiation of mouse erythroleukemia cells by genistein, an inhibitor of tyrosine protein kinases. Cancer Res. 1991;51:764-768. [PubMed] |
| 18. | Miller DR, Lee GM, Maness PF. Increased neurite outgrowth induced by inhibition of protein tyrosine kinase activity in PC12 pheochromocytoma cells. J Neurochem. 1993;60:2134-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Simon HU, Yousefi S, Blaser K. Tyrosine phosphorylation regulates activation and inhibition of apoptosis in human eosinophils and neutrophils. Int Arch Allergy Immunol. 1995;107:338-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Huang P, Robertson LE, Wright S, Plunkett W. High molecular weight DNA fragmentation: a critical event in nucleoside analogue-induced apoptosis in leukemia cells. Clin Cancer Res. 1995;1:1005-1013. [PubMed] |
| 21. | Xu LH, Owens LV, Sturge GC, Yang X, Liu ET, Craven RJ, Cance WG. Attenuation of the expression of the focal adhesion kinase induces apoptosis in tumor cells. Cell Growth Differ. 1996;7:413-418. [PubMed] |
| 22. | Davis JN, Singh B, Bhuiyan M, Sarkar FH. Genistein-induced upregulation of p21WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr Cancer. 1998;32:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 317] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 24. | van der Woude CJ, Jansen PL, Tiebosch AT, Beuving A, Homan M, Kleibeuker JH, Moshage H. Expression of apoptosis-related proteins in Barrett's metaplasia-dysplasia-carcinoma sequence: a switch to a more resistant phenotype. Hum Pathol. 2002;33:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Panaretakis T, Pokrovskaja K, Shoshan MC, Grandér D. Activation of Bak, Bax, and BH3-only proteins in the apoptotic response to doxorubicin. J Biol Chem. 2002;277:44317-44326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Bellosillo B, Villamor N, López-Guillermo A, Marcé S, Bosch F, Campo E, Montserrat E, Colomer D. Spontaneous and drug-induced apoptosis is mediated by conformational changes of Bax and Bak in B-cell chronic lymphocytic leukemia. Blood. 2002;100:1810-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Matter-Reissmann UB, Forte P, Schneider MK, Filgueira L, Groscurth P, Seebach JD. Xenogeneic human NK cytotoxicity against porcine endothelial cells is perforin/granzyme B dependent and not inhibited by Bcl-2 overexpression. Xenotransplantation. 2002;9:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Lanzi C, Cassinelli G, Cuccuru G, Supino R, Zuco V, Ferlini C, Scambia G, Zunino F. Cell cycle checkpoint efficiency and cellular response to paclitaxel in prostate cancer cells. Prostate. 2001;48:254-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Mertens HJ, Heineman MJ, Evers JL. The expression of apoptosis-related proteins Bcl-2 and Ki67 in endometrium of ovulatory menstrual cycles. Gynecol Obstet Invest. 2002;53:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Mehta U, Kang BP, Bansal G, Bansal MP. Studies of apoptosis and bcl-2 in experimental atherosclerosis in rabbit and influence of selenium supplementation. Gen Physiol Biophys. 2002;21:15-29. [PubMed] |
| 31. | Chang WK, Yang KD, Chuang H, Jan JT, Shaio MF. Glutamine protects activated human T cells from apoptosis by up-regulating glutathione and Bcl-2 levels. Clin Immunol. 2002;104:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Chen GG, Lai PB, Hu X, Lam IK, Chak EC, Chun YS, Lau WY. Negative correlation between the ratio of Bax to Bcl-2 and the size of tumor treated by culture supernatants from Kupffer cells. Clin Exp Metastasis. 2002;19:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Usuda J, Chiu SM, Azizuddin K, Xue LY, Lam M, Nieminen AL, Oleinick NL. Promotion of photodynamic therapy-induced apoptosis by the mitochondrial protein Smac/DIABLO: dependence on Bax. Photochem Photobiol. 2002;76:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Sun F, Akazawa S, Sugahara K, Kamihira S, Kawasaki E, Eguchi K, Koji T. Apoptosis in normal rat embryo tissues during early organogenesis: the possible involvement of Bax and Bcl-2. Arch Histol Cytol. 2002;65:145-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Jang MH, Shin MC, Shin HS, Kim KH, Park HJ, Kim EH, Kim CJ. Alcohol induces apoptosis in TM3 mouse Leydig cells via bax-dependent caspase-3 activation. Eur J Pharmacol. 2002;449:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Tilli CM, Stavast-Koey AJ, Ramaekers FC, Neumann HA. Bax expression and growth behavior of basal cell carcinomas. J Cutan Pathol. 2002;29:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Pettersson F, Dalgleish AG, Bissonnette RP, Colston KW. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-gamma and altered expression of Bcl-2/Bax. Br J Cancer. 2002;87:555-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |