Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1726
Revised: November 29, 2003
Accepted: December 6, 2003
Published online: June 15, 2004
AIM: To investigate the effects of mifepristone on the invasive and metastatic potential of human gastric adenocarcinoma cell line MKN-45 and its mechanisms.
METHODS: After incubation with various concentrations of mifepristone (5, 10, 20 μmol/L), the adhesion to artificial basement membrane, Matrigel, and the migration of MKN-45 cells were assayed using MTT assay and Transwell cell culture chambers, respectively. Enzyme- linked immunoabsorbent assay (ELISA) and flow cytometry were used to determine the expression of vascular endothelial growth factor (VEGF) and integrin β3 in the cells. After subcutaneous transplantation of MKN-45 cells in nude mice, mifepristone (50 mg/kg·d) was administrated subcutaneously for 8 wk to assess its effects on tumor metastasis. Immunohistochemical analysis was used to detect the expression of VEGF and microvascular density (MVD) in xenografted tumors.
RESULTS: Mifepristone dose-dependently inhibited the heterotypic adhesion to Matrigel of MKN-45 cells. The inhibition was accompanied by a significant down-regulation of integrin β3 expression in the cells. After incubation with 5, 10, 20 μmol/L mifepristone, the number of migrated MKN-45 cells was 72 ± 8, 50 ± 6, 41 ± 5 in experiment group, and 94 ± 16 in control group (P < 0.01). Meanwhile, secreted VEGF protein of MKN-45 cells in mifepristone-treated group (14.2 ± 2.9, 8.9 ± 3.1, 5.4 ± 2.1 ng/g per liter) was significantly lower than that in control group (22.7 ± 4.3 ng/g per liter, P < 0.01). In vivo, mifepristone decreased the number of metastatic foci in lungs of nude mice and down-regulated the expression of VEGF and MVD in the xenograted tumors.
CONCLUSION: Mifepristone can effectively inhibit the invasive and metastatic potential of human gastric adenocarcinoma cell line MKN-45 in vitro and in vivo through inhibition of heterotypic adhesion to basement membrane, cell migration and angiogenesis.
-
Citation: Li DQ, Wang ZB, Bai J, Zhao J, Wang Y, Hu K, Du YH. Effects of mifepristone on invasive and metastatic potential of human gastric adenocarcinoma cell line MKN-45
in vitro andin vivo . World J Gastroenterol 2004; 10(12): 1726-1729 - URL: https://www.wjgnet.com/1007-9327/full/v10/i12/1726.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i12.1726
Mifepristone is a progesterone receptor (PR) antagonist that has been widely used as the first-line drug for the termination of early pregnancy[1]. Recently, considerable studies[2,3] have proved that mifepristone exerts markedly anti-tumor effects on PR-positive tumor cells, such as breast cancer and ovarian cancer, without obvious side-effects and drug resistance. Its similar effect on PR-positive human gastric adenocarcinoma cell line MKN-45 was also demonstrated in our laboratory.
More interestingly, accumulating evidences show that embryo implantation and tumor metastasis share striking similarities in biological behaviors, such as cell adhesion[4], immune escape[5], angiogenesis[6], invasion[7] and tumor metastasis-related gene expression[8]. By this rational, there is an increasing interest in addressing the role of mifepristone, an agent against embryo implantation, in anti-tumor invasion and metastasis. Therefore, the present study was undertaken to further investigate the effects of mifepristone on the invasive and metastatic potential of MKN-45 cells in vitro and in vivo and its possible mechanisms.
Human gastric adenocarcinoma cell line MKN-45 was obtained from Wuhan University Type Culture Collection (Wuhan, China), and maintained in phenol red-free RPMI1640 (Gibco BRL, Grand Island, NY) supplemented with 100 mL/L1 fetal bovine serum (Hyclone, Logan, UT), 105 U/L penicillin and 100 mg/L streptomycin at 37 °C in a humidified atmosphere containing 50 mL/L CO2 in air. When cells were grown to approximately 50% confluence, the medium was then replaced with serum-free RPMI1640. After 24 h, fresh media containing 5, 10, 20 μmol/L mifepristone (Sigma Chemical Co., St Louis, MO) were added, respectively. Control cells were treated with the same volumes of vehicle (ethanol). Unless otherwise stated, the cells were harvested after 96 h of incubation.
Each well in 96-well tissue culture plates was coated with 2 μg of Matrigel (Collaborative Research Inc., Bedford, MA) and allowed to dry in a laminar flow cabinet overnight at room temperature. After washed three times with PBS to remove excess and unbound Matrigel, the wells were blocked with 20 μL of a 20 mg/L bovine serum albumin (BSA, Sigma) solution in RPMI1640 medium for 1 h at 37 °C. Aliquots of 8×104 cells, in 100 μL of serum-free RPMI1640 medium containing various concentrations of mifepristone, were added to each well and the cells were allowed to adhere for 1 h at 37 °C. When the incubation was completed, the wells were washed three times with PBS to remove unbound cells. Then, the remaining cells were continuously incubated with MTT solutions (40 μg/well) for 4 h at 37 °C, followed by treatment with 200 μL of dimethyl sulfoxide (DMSO) for 10 min.
Finally, A570nm of each well was measured using an ELISA plate reader (Bio-Rad, USA). Results were expressed as the adhesive rate (%) that was calculated according to the following formula: (A570nm of the adhered cells/A570nm of total cells)×100%.
The harvested cells were fixed with 40 mg/L paraformaldehyde for 10 min, followed by treatment with 2 mg/L Triton X-100 for 10 min. After treatment with normal rabbit serum for 10 min to block non-specific binding, the cells were incubated for 1 h at 4 °C with mouse anti-human monoclonal antibody against β3 (Santa Cruz, USA), followed by treatment with FITC-conjugated goat anti-mouse IgG (Vector, Burlingame, CA) for 30 min at 4 °C. The percentage of positive cells was determined using the FACS Calibur flow cytometry (Becton & Dickinson) with an excitation wavelength of 488 nm.
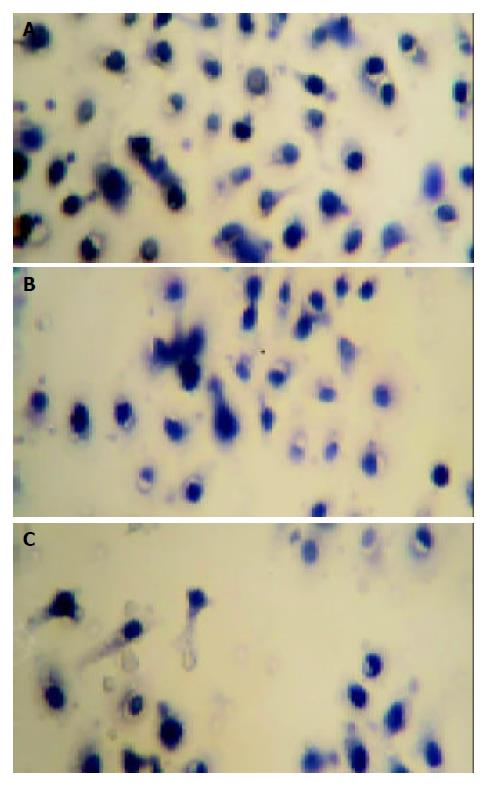
Migration of MKN-45 cells was assayed in Transwell cell culture chambers with 6.5-mm-diameter polycarbonate membrane filters containing 8-μm-pore size (Becton Dickinson Labware, Bedford, MA). Fibroblast-conditioned medium, obtained from confluent NIH 3T3 cell cultures in serum-free RPMI 1640, was used as the chemoattractant and added to the lower wells of the chambers. Aliquots of 2×104 cells in 300 μL fresh medium containing various concentrations of mifepristone were seeded into the upper wells of the cell inserts. After 24 h of incubation at 37 °C, the non-migrating cells were removed from the upper surface of the membrane with a cotton swab. The cells on the lower surface of the membrane were fixed with ice-cold methanol and then stained with haematoxylin and eosin. The number of migrated cells was counted under a light microscope. Five random microscopic fields (×400) were counted per well and the mean was determined.
The media were collected after 48 h for VEGF ELISA determinations as described below. The cells were taken through three freeze-thaw cycles, centrifuged and supernatant was collected for determination of protein concentration as described previously. VEGF levels in the cell culture media were measured using a Quantikine kit from R & D Diagnostics (Minneapolis, MN) using the procedure provided by the supplier. Human recombinant VEGF included in the kit was used to construct a standard curve and obtain absolute values of VEGF protein content. The values were then normalized to the total protein concentration in each dish.
Two×107 MKN-45 cells were subcutaneously xenografted in the right flank of 8-wk-old male BALB/c-nu/nu mice (Shanghai Experimental Animal Center, Chinese Academy of Sciences, China). When tumors reached a mean volume of 100 mm3, mice were randomly divided into two groups (8 mice in per group) and treated as the following. Mice in experiment group were administrated subcutaneously with mifepristone at the dose of 50 mg/kg·d, whereas mice in control were subcutaneouely injected with saline every day. After 8 wk of treatment, lungs were harvested, and the weights of lungs were determined. The number of metastatic foci in lungs fixed with Bouin’s solution for 24 h was counted under a stereomicroscope. Meanwhile, the tumors were resected, fixed with 40 g/L formaldehyde in PBS, embedded in paraffin and sliced into 4-μm-thick sections for immunohistochemical analysis.
The expression of VEGF and MVD in harvested tumors was determined immunohisto-chemically using an avidin-biotin-peroxidase complex (ABC) kit (Vector Laboratories, USA). Unless otherwise stated, all steps were performed at room temperature. Briefly, after removal of wax, tissue sections were treated with 30 mL/L hydrogen peroxidase for 30 min to block endogenous peroxidase activity, and microwaved in 10 mmol/L citrate buffer for 10 min to retrieve antigens. After blocked with normal goat serum, rabbit anti-human polyclonal antibody against VEGF and factor VIII-related antigen (Santa Cruz Biotechnology, USA) at a 100-fold dilution were separately applied to sections and incubated overnight at 4 °C. This was followed by treatment with biotin-labeled goat anti-rabbit IgG for 60 min. The ABC complex was added and allowed to stand for 30 min. Sites of immunoreaction were visualized with 3, 3’-diaminobenzidine (DAB), followed by counterstaining with Mayer’s haematoxylin, if necessary. Between each step, the sections were washed three times with 100 mmol/L Tris-HCl buffer containing 0.1 mg/L Triton X-100. For a positive control, breast cancer tissue was used and the primary antibody was replaced by normal rabbit serum as a negative control.
Data were expressed as mean ± SD. Statistical analysis was performed using the Student’s t test and chi-square test. P < 0.05 was considered statistically significant.
MTT assay revealed that mifepristone dose-dependently inhibited the cell adhesion to artificial basement membrane, Matrigel. The adhesive rate of MKN-45 cells was 78.2 ± 5.0%, 65.4 ± 4.7%, 49.8 ± 4.2% in mifepristone-treated group, and 85.6 ± 6.3% in control group (P < 0.05). To further explore whether the change of cell adhesion molecule expression in MKN-45 cells contributed to the inhibition, flow cytometry was used to detect the expression of integrin β3 in the cells. Results showed that the expression of integrin β3 in MKN-45 cells treated with 5, 10, 20 μmol/L mifepristone was significantly lower than that in control group (25.4 ± 3.6%, 19.6 ± 2.9%, 15.8 ± 2.2% vs 31.8 ± 4.1%, P < 0.05).
The effect of mifepristone on the migration of MKN-45 cells was evaluated in the Transwell cell culture chambers. As shown in Figure 1, there was a significant decrease in the number of migrated MKN-45 cells in mifepristone-treated group (72 ± 8, 50 ± 6, 41 ± 5) as compared with that in control group (94 ± 16, P < 0.01).
After treatment with 5, 10, 20 μmol/L mifepristone for 48 h, secreted VEGF protein in the cell culture media measured by ELISA assay, was 14.2 ± 2.9, 8.9 ± 3.1 and 5.4 ± 2.1 ng/g per liter, respectively. There was a significant difference in VEGF expression as compared with that in the control cells (22.7 ± 4.3 ng/g per liter, P < 0.01).
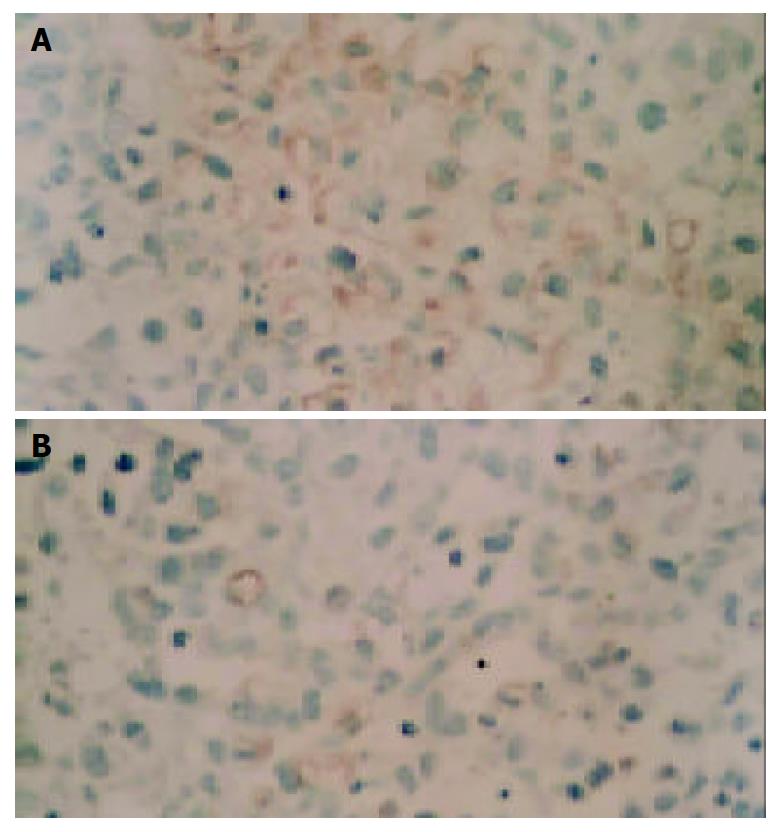
During necropsy, lung metastasis of gastric cancer was found in 6 mice of the control group, and in 4 mice of mifepristone group (Figure 2). As far as the number of metastatic foci in lungs was concerned, there was a significant difference in mifepristone-treated group as compared with control group (8 ± 2 vs 18 ± 7, P < 0.05). In addition, the weights of xenografted tumors in nude mice treated with mifepristone (201 ± 36 mg) were significantly lower than those in control group (298 ± 54 mg, P < 0.05). To evaluate the effects of mifepristone on MVD and VEGF expression in xenografted tumors, immnunohistochemical staining was performed using ABC method. Results revealed that VEGF was highly expressed in xenografted gastric cancer in nude mice (Figure 3A). After treatment with mifepristone for 8 wk, the expression of VEGF was markedly down-regualted in the tumors (Figure 3B). In addition, MVD of xenografted tumors was (11.2 ± 2.5)/400×visual field in mifepristone-treated group, and (29.8 ± 7.6)/400×in the control group (P < 0.01).
Although considerable evidence[9,10], both clinical and experimental, has demonstrated that mifepristone exerts markedly anti-proliferative effects on PR-positive malignant tumor cells, the role of mifepristone in anti-tumor invasion and metastasis, especially on gastric cancer, is poorly understood. In the presnet study, we demonstrated that mifepristone effectively inhibited the invasive and metastatic potential of human gastric adenocarcinoma cell line MKN-45 in vitro and in vivo through multiple mechanisms.
For tumor cells, increase of heterotypic adhesion to basement membrane and decrease of homotypic adhesion to the same cells have been defined as the critical event of tumor invasion that signals the initiation of metastatic cascade[11,12]. In the present study, heterotypic adhesion of MKN-45 cells to artificial basement membrane, Matrigel, was examined with MTT dye assay to stain the adhered cells. Results showed that mifepristone dose-dependently decreased the adhesive rate of MKN-45 cells. Moreover, the findings were further proved by the down-regulation of integrin β3 expression in the cells treated with mifepristone.
Integrin β3, one member of integrins superfamily, plays a fundamental role in tumor cell heterotypic adhesion to extracellular matrix and basement membrane, which has been found to be mediated through a specific arginine-glycine-aspartic acid (RGD) amino acid sequence[13]. Brakebusch et al[14] reported that high expression of integrin β3 directly correlated with tumorigenicity and tumor progression. To further explore whether the change of integrin β3 expression in MKN-45 cells contributed to the inhibitory effect of mifepristone on cell adhesion, flow cytometry was perfomed. We found that mifepristone down-regulated the expression of integrin β3 in MKN-45 cells in a dose-dependent manner. The results were also demonstrated by the work of Li et al[15] who reported that mifepristone significantly down-regulated the expression of integrin β3 in decidua and chorionic villi of early pregnancy, and the effect might be related with the anti-pregnancy mechanism of mifepristone. Taken together, the inhibitory effect of mifepristone on heterotypic adhesion of MKN-45 cells may be associated with the down-regulation of integrin β3. Moreover, integrin β3 has been found to function not only as a cell adhesion molecule, but also as a signaling molecule for regulation of angiogenesis[16]. Therefore, down-regulation of integrin β3 expression in MKN-45 cells may be partially responsible for the inhibition of angiogenesis in the cells by mifepristone.
Continuous growth, invasion and metastasis of malignant tumors including human gastric cancer are dependent on angiogenesis factors regulated by peptide growth factors, of which VEGF is one of the most selective and potent[17]. Hyder et al[18] reported that progestins could induce the expression of VEGF mRNA and protein in human breast cancer cell line T47D, and this effect was blocked by the antiprogestin agent mifepristone. The finding suggests that mifepristone may be useful to inhibit proliferation and metastasis in some tumors by blocking VEGF production. Thus, there is a great interest in exploring the effect of mifepristone on the expression of VEGF in MKN-45 cells. After exposure of MKN-45 cells to various concentrations of mifepristone for 48 h, a dose-dependent decrease in the media levels of VEGF was observed. Furthermore, the results were further supported by animal experiments. Immunohistochemical analysis showed that mifepristone significantly down-regulated the expression of VEGF and MVD in xenografted gastric cancer in nude mice. Summarily, it seems reasonable to conclude that the inhibitory effect of mifepristone on the invasive and metastatic potential of MKN-45 cells is medicated partially via blocking VEGF production.
On the other hand, tumor cell migration was necessary at the initiation of metastatic cascade, when the tumor cells left the primary site and gained access to the circulation and also at the end of invasion, when they were entering the secondary site[19]. Theoretically, the decrease of tumor cell migration would contribute to the inhibition of tumor invasion and metastasis. In the study, the effect of mifepristone on the migaration of MKN-45 cells was estimated in Transwell cell culture chambers. Results showed that a significant decrease in the number of migrated MKN-45 cells was observed in mifepristone-treated group as compared with control group.
In summary, mifepristone effectively inhibited the invasive and metastatic potential of human MKN-45 gastric adenocarcinoma cells through inhibition of the heterotypic adhesion to basement membrane, cell migration and angiogenesis. These findings, linked to its anti-proliferative effects, indicate that mifepristone may be a beneficial agent for additional and complementary use in the management of gastric cancer.
Edited by Xu FM and Wang XL
| 1. | Mahajan DK, London SN. Mifepristone (RU486): a review. Fertil Steril. 1997;68:967-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Rocereto TF, Saul HM, Aikins JA, Paulson J. Phase II study of mifepristone (RU486) in refractory ovarian cancer. Gynecol Oncol. 2000;77:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Thomas M, Monet JD. Combined effects of RU486 and tamoxifen on the growth and cell cycle phases of the MCF-7 cell line. J Clin Endocrinol Metab. 1992;75:865-870. [PubMed] |
| 4. | Lash GE, Fitzpatrick TE, Graham CH. Effect of hypoxia on cellular adhesion to vitronectin and fibronectin. Biochem Biophys Res Commun. 2001;287:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Arck PC, Hertwig K, Hagen E, Hildebrandt M, Klapp BF. Pregnancy as a model of controlled invasion might be attributed to the ratio of CD3/CD8 to CD56. Am J Reprod Immunol. 2000;44:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Murray MJ, Lessey BA. Embryo implantation and tumor metastasis: common pathways of invasion and angiogenesis. Semin Reprod Endocrinol. 1999;17:275-290. [PubMed] |
| 7. | Fitzpatrick TE, Lash GE, Yanaihara A, Charnock-Jones DS, Macdonald-Goodfellow SK, Graham CH. Inhibition of breast carcinoma and trophoblast cell invasiveness by vascular endothelial growth factor. Exp Cell Res. 2003;283:247-255. [PubMed] |
| 8. | Janneau JL, Maldonado-Estrada J, Tachdjian G, Miran I, Motté N, Saulnier P, Sabourin JC, Coté JF, Simon B, Frydman R. Transcriptional expression of genes involved in cell invasion and migration by normal and tumoral trophoblast cells. J Clin Endocrinol Metab. 2002;87:5336-5339. [PubMed] |
| 9. | Yokoyama Y, Shinohara A, Takahashi Y, Wan X, Takahashi S, Niwa K, Tamaya T. Synergistic effects of danazol and mifepristone on the cytotoxicity of UCN-01 in hormone-responsive breast cancer cells. Anticancer Res. 2000;20:3131-3135. [PubMed] |
| 10. | El Etreby MF, Liang Y, Lewis RW. Induction of apoptosis by mifepristone and tamoxifen in human LNCaP prostate cancer cells in culture. Prostate. 2000;43:31-42. [PubMed] [DOI] [Full Text] |
| 11. | Stetler-Stevenson WG, Kleiner DE. Molecular biology of cancer: invasion and metastases. Cancer principles and practice of oncology. 6th ed. Philadelphia: Lippincott Williams Willkins 2001; 123-136. |
| 12. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19489] [Article Influence: 779.6] [Reference Citation Analysis (0)] |
| 13. | Liotta LA, Kohn EC. Invasion and metastases. Cancer medicine. 5th ed. Baltimore: Williams Wilkins 2001; 121-131. |
| 14. | Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fässler R. Integrins in invasive growth. J Clin Invest. 2002;109:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Li RZ, Wang ZH, Wu RF, Lu SY, Shi B. Expression of integrin b3 and extracellular matrix: fibronectin and laminin in deciduas and chorionic chill of medical abortion for early pregnancy. J Reprod Med. 1999;8:214-216. |
| 16. | Kohn EC, Liotta LA. Metastasis and angiogenesis: molecular dis-section and noval applications. eds. The molecular basis of cancer. 2nd ed. Philadephia: W.B Saunders Company 2001; 163-172. |
| 17. | Folkman J. Tumor angiogenesis. Clinical oncology. 2nd ed. Orlando: Harcourt Publishers Limited 2001; 132-151. |
| 18. | Hyder SM, Chiappetta C, Stancel GM. Pharmacological and endogenous progestins induce vascular endothelial growth factor expression in human breast cancer cells. Int J Cancer. 2001;92:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Fidler IJ. Cancer biology: invasion and metastasis. Clinical oncology. 2nd ed. Orlando: Harcourt Publishers Limited 2001; 29-53. |