Published online May 15, 2004. doi: 10.3748/wjg.v10.i10.1457
Revised: August 11, 2003
Accepted: September 18, 2003
Published online: May 15, 2004
AIM: To observe the effect of tail vein injection with donor hepatocytes and/or splenocytes on the islet xenotransplantation rejection.
METHODS: New-born male pigs and BALB/C mice were selected as donors and recipients respectively. Islet xenotransplantation was performed in recipients just after the third time of tail vein injection with donor hepatocytes and/or splenocytes. Macrophage phagocytosis, NK(natural killing cell) killing activity, T lymphocyte transforming function of spleen cells, antibody forming function of B lymphocytes, and T lymphocyte subsets were taken to monitor transplantation rejection. The effects of this kind of transplantation were indicated as variation of blood glucose and survival days of recipients.
RESULTS: The results showed that streptozotocin (STZ) could induce diabetes mellitus models of mice. The pre-injection of donor hepatocytes, splenocytes or their mixture by tail vein injection was effective in preventing donor islet transplantation from rejection, which was demonstrated by the above-mentioned immunological marks. Each group of transplantation could decrease blood glucose in recipients and increase survival days. Pre-injection of mixture of donor hepatocytes and splenocytes was more effective in preventing rejection as compared with that of donor hepatocyte or splenocyte pre-injection respectively.
CONCLUSION: Pre-injection of donor hepatocytes, splenocytes or their mixture before donor islet transplantation is a good way in preventing rejection.
- Citation: Tang TH, Li CL, Li X, Jiang FQ, Zhang YK, Ren HQ, Su SS, Jiang GS. Immune tolerance in pancreatic islet xenotransplantation. World J Gastroenterol 2004; 10(10): 1457-1461
- URL: https://www.wjgnet.com/1007-9327/full/v10/i10/1457.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i10.1457
Insulin dependent diabetes mellitus (IDDM) so far is treated with a constant low dose insulin injection and immunosuppressive agents. But a large quantity of clinical data indicated that long-term insulin injection could induce insulin resistance and was not favorable to prevent some serious complications[1]. Long-term application of immunosuppressive drugs, for example, high-dose cyclosporine and tacrolimus, could bring about toxic effects on islets and normal liver and kidney[2,3]. So in some cases, it is limited to use immunosuppressive drugs for a long period at high dose. However, islet transplantation has the potential to cure diabetes mellitus[4], especially insulin-dependent diabetes mellitus[5]. Islet transplantation could make insulin maintain normal level of blood glucose and prevent serious complications. In general, islet allotransplantation is safe and efficient to reduce hyperglycemia, and leads to insulin independence in patients with insulin dependent diabetes[6,7]. But this kind of islets source is limited, which becomes the chief obstacle for treating patients. So it is necessary to find alternative islet sources, such as xenotransplantation of islets of new-born pigs. However, the rejection for xenotransplantation should be mornitored efficiently. Usually, the way of preventing rejection lies in using high dose of immunosuppressive drugs, which gives rise to serious side effects. On the other hand, pre-injection of a suitable number of the same donor lymphocytes or blood cells could induce immune tolerance in different kinds of organ transplantations[8-14]. Some other laboratories also reported that immunologic isolation, or transplantation of microencapsulated islets, could reduce rejection of islet xenotransplantation[15,16]. In the present study, pig islet xenotransplantation in mice was performed after injection of donor hepatocytes, splenocytes or their mixture.
Eighty-four BALB/C female mice (22-24 g) and 6 new born male pigs were used as recipients and donors respectively. Each BALB/C mouse was injected 0.18-0.2 mL streptozotocin (STZ) (220 mg/kg) via tail vein. When blood glucose was increased to 11.1 mmol/L, the diabetes mellitus model of mice was used in the present experiments.
Under sterile condition, Pig pancreases were washed three times with Hanks’ solution. Trypsin digestion method was used to isolate single β cells or islets, referring to the method reported by Heiser[17]. Activity of β cells was evaluated by dithiozon (DTZ) staining and the activity should be equal or over 90%. Under bacteria-free condition, pig livers or spleens were washed three times with serum-free RPMI1640, then ground on a 200# stainless steel net to make single cell suspension, washing two times (2000 rpm/min, 5 min) with normal saline (N.S). The number of cells was adjusted to 2 × 107/mL. The cell viability was detected by Hoechst/propidium iodine (Ho/PI), both hepatocytes and splenocytes were used as donor cells with viability of more than 95%.
For hepatocyte injection group, 0.2 mL liver single cell suspension was injected into diabetic mice via tail vein every 24 h for 3 times. After the last injection of donor hepatocytes, 0.5 mL pig islets (the number of islets was 980) was transplanted into peritoneal of mouse recipient.
For splenocyte injection group, 0.2 mL spleen single cell suspension was injected into diabetes mellitus mice via tail vein every 24 h for 3 times. After the last time of injection, 0.5 mL pig islets (the number of islets was 980) was transplanted into peritoneal mouse recipient.
For mixture injection group, 0.1 mL single hepatocyte cell suspension and 0.1 mL splenocyte single cell suspension were injected into model mice via tail vein every 24 h for 3 times. After the last time of injection, 0.5 mL pig islets (the number of islets was 980) was transplanted into their peritoneal.
For islets group, 0.2 mL serum-free RPMI1640 was injected into model mice via tail vein every 24 h for 3 times. After the last time of injection, 0.5 mL pig islets (the number of islets was 980) was transplanted into recipient peritoneal. Three mice in parallel were measured for each mark and the result was indicated as an average. Twelve mice in each group were observed in terms of their living days and survival rate.
The level of blood glucose in mice was measured every three days by a Glucose monitoring system before and after islet transplantation. The level of blood glucose in model mice before transplantation should be over 11.1 mmol/L. The survival of transplanted-islets was indicated as at least decreasing 2-fold as compared with that before transplantation, which was indicated as functional surviving. Rejection was monitored by the level of blood glucose in mice after islet transplantation with more than 16.5 mmol/L for a continuation of 2 times.
Candida albicans were used as targets, constant method in our laboratory was taken to detect phagocytosis of macrophages in abdominal cavity of mice. Phagocytosis percent = (phagocytosis of macrophages/total macrophages) × 100%, Phagocytosis index = (number of phagocytotic candida albicans in 100 macrophages/total macrophages) × 100%.
Pig spleens were washed three times with serum-free RPMI1640. The spleens were ground on a 200# stainless steel net. The number of cells was adjusted to 1 × 106/mL with RPMI1640. Each well of the culture plate was added 100 μL cell suspension, 6 wells in parallel, 3 wells were added conA solution 50 μL, the other 3 wells were added RPMI1640 50 μL as control. The cells were incubated in 50 mL/L CO2, at 37 °C for 56 h, then 3H-TdR 1 μCi/well was added and cultured cells for 72 h. Cpm value was detected with a β-liquid scintillation counter. Transformation function was evaluated as average Cpm value.
For effector cells, mice spleens were made into single cell suspension as the same as above-mentioned method, and the cell density was adjusted to 1 × 107/mL in 100 mL/L fetal calf serum(FCS) RPMI1640 medium. For target cells, exponentially growing YAC-1 cells were adjusted to 2 × 105/mL cell suspension with 100 mL/L FCS RPMI1640. A 100 μL effector cell suspension and 100 μL target cell suspension were added into each well respectively in a 40-well plate as experimental group, 3 wells in parallel. On the other hand, 100 μL/well effector cells and 100 μL/well RPMI1640 were used as effector group, 100 μL 100 mL/L FCS RPMI1640 and 100 uL target cells as target group. They were cultured them in 50 mL/L CO2, at 37 °C for 24 h. Then 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide(MTT) 20 μL/well, continually cultured for another 4 h, centrifuged at 2000 rpm/min 10 min, the supernatant was discarded, add 1000 g/L dimethyl sulfoxide (MDS) 100 μL was added into each well, cells were lysed with vibration. After 10 min, A value was measured at 570 nm with an ELISA reader. NK killing activity = [1-(Experimental A-Effector A)/TargetA] × 100%.
The total number of lymphocytes was calculated directly under microscopy. T lymphocytes and its subpopulations were measured by constant strept avidin-biotin complex (SABC) staining. SABC kit was supplied by Wuhan Boshide Company.
Quantitative hemolytic spectrometry (QHS) was used to run antibody-forming assay. Mice spleens were washed three times with normal saline. A 200# stainless steel net was used to grind spleen tissues into single cell suspension. The cells were re-suspended and adjusted to a final concentration of 2 × 107/mL Hanks buffer (pH7.2). A 1 mL cell suspension and 1 mL 2 mL/L sheep red blood cell (SRBC) (1.5 × 108/mL cells), then 1:10 complement 1 mL was added at 37 °C for 1 h, centrifuged at 3000 rpm/min for 5 min, the A value of supernatant at 413 nm detected by 721 spectrophotometer. A value was indicated as the ability of antibody formation of B lymphocytes in vitro.
Pathologic changes including inflammatory infiltration of local site of islet transplantation in different experimental groups were examined.
The experiments were repeated 3 times and the data were expressed as mean ± SE, and significant difference was assessed by Student’s t test.
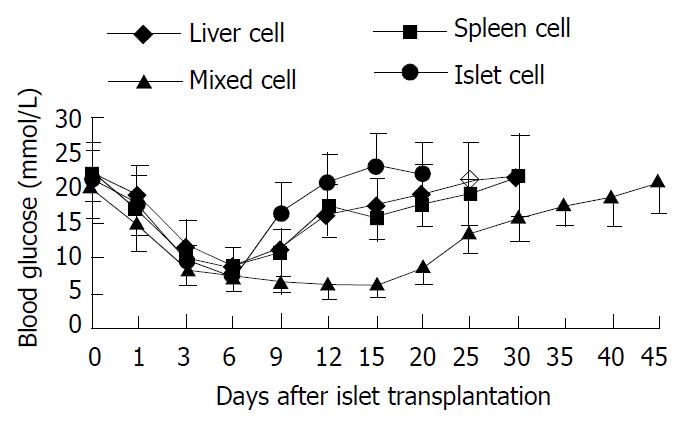
The Variations of blood glucose level after islet transplantation is shown in Figure 1.
The survival time of hepatocyte injection group, splenocyte injection group, mixture group and islet group was 26.42 ± 5.87 d, 29.56 ± 4.52 d, 43.00 ± 4.55 d and 16.92 ± 2.47 d respectively (survival time of diabetic model mice was 3.86 ± 0.85 d). The survival time of islet transplantation group was shorter than any other experimental group (P < 0.05). So pre-injection of hepatocytes, splenocytes or their mixture from the same donor was effective in preventing rejection and delaying the time of death.
Phagocytosis of macrophages in diabetic mice after pig islet xenotransplantation is summarized in Table 1.
| Group | Pre-treatment | 7 d post-treatment | 14 d post-treatment | |||
| Percent (%) | Index | Percent (%) | Index | Percent (%) | Index | |
| Hepatocytes group | 59.3 ± 4.5 | 1.05 ± 0.04 | 62.8 ± 4.37 | 1.28 ± 0.13 | 70.6 ± 3.82 | 1.25 ± 0.07 |
| Splenocytes group | 62.5 ± 3.0 | 1.04 ± 0.07 | 63.1 ± 6.45 | 1.18 ± 0.14 | 69.5 ± 3.62 | 1.21 ± 0.09 |
| Mixture group | 63.1 ± 3.8 | 1.07 ± 0.06 | 61.6 ± 3.54 | 1.12 ± 0.09 | 62.8 ± 4.70 | 1.09 ± 0.08 |
| Islet group | 62.0 ± 3.3 | 1.06 ± 0.08 | 73.5 ± 5.51 | 1.41 ± 0.18 | 72.7 ± 5.71 | 1.47 ± 0.10 |
Table 1 shows that phagocytosis percentage of islet group was increased, and the phagocytosis index of islet or hepatocyte group was obviously increased (P < 0.05). There was no significant variation of phagocytosis percentage or index in other transplantation groups that received pre-injection of hepatocytes or mixture of hepatocytes and splenocytes 7 d after islet transplantation (P < 0.05). After 14 d, phagocytosis percentage in hepatocyte, splenocyte and islet groups was significantly increased (P < 0.05), phagocytosis index of islet or hepatocyte group was significantly increased (P < 0.05).
Seven days after transplantation, the cpm value of spleen lymphocytes in recipients of hepatocyte group, splenocyte group, mixture group or islet group was higher after pig islet xenotransplantation than before transplantation (P < 0.05). After 14 d, the value of hepatocyte group or islet group was still higher than that before transplantation (P < 0.05). But there was no significant difference between splenocyte group and mixture group (P > 0.05) (Table 2).
| Group | Pre-transplantation (cpm) | 7 d post-transplantation (cpm) | 14 d post-transplantation (cpm) |
| Hepatocytes group | 11437.56 ± 1137.55 | 23453.74 ± 4157.28 | 20178.56 ± 4159.74 |
| Splenocytes group | 11437.56 ± 1137.55 | 25834.66 ± 3763.37 | 15336.25 ± 3782.38 |
| Mixture group | 11437.56 ± 1137.55 | 21920.05 ± 600.08 | 14345.53 ± 2653.28 |
| Islets group | 11437.56 ± 1137.55 | 32339.13 ± 5071.67 | 22182.16 ± 750.28 |
NK activity of each experimental group, as compared with that before transplantation, was increased 7 d after islet transplantation (P < 0.05), and their NK activity was continually higher than that before transplantation even 14 d after transplantation (P < 0.05). As to the mixture group, there was no significant increase of NK activity 7 or 14 d after islet transplantation as compared with that before islet transplantation (P > 0.05) (Table 3).
| Group | Pre-treatment | 7 d post-treatment | 14 d post-treatment |
| Hepatocytes group | 31.56 ± 4.23 | 63.17 ± 3.47 | 60.26 ± 7.65 |
| Splenocytes group | 31.76 ± 3.48 | 62.50 ± 4.86 | 56.19 ± 8.13 |
| Mixture group | 31.37 ± 8.09 | 48.00 ± 12.0 | 34.33 ± 14.57 |
| Islet group | 35.67 ± 3.51 | 76.23 ± 12.50 | 71.00 ± 3.0 |
| Group | Pretreatment(%) | 7 d post-treatment (%) | 14 d post-treatment (%) | ||||||
| CD3 | CD4 | CD8 | CD3 | CD4 | CD8 | CD3 | CD4 | CD8 | |
| Hepatocyte group | 61.6 ± 4.5 | 42.0 ± 4.6 | 24.3 ± 1.5 | 72.7 ± 5.5 | 51.6 ± 4.5 | 28.0 ± 3.6 | 70.0 ± 2.0 | 52.7 ± 4.9 | 26.7 ± 4.2 |
| Splenocyte group | 66.0 ± 3.6 | 46.3 ± 4.0 | 24.6 ± 2.5 | 73.0 ± 4.4 | 53.3 ± 4.5 | 31.3 ± 4.1 | 68.7 ± 5.7 | 45.3 ± 4.1 | 33.0 ± 3.6 |
| Mixture group | 64.7 ± 2.5 | 44.3 ± 6.7 | 25.3 ± 4.9 | 65.0 ± 5.3 | 47.6 ± 3.8 | 28.3 ± 2.5 | 61.6 ± 3.5 | 47.0 ± 4.4 | 27.7 ± 2.1 |
| Islet group | 65.0 ± 7.0 | 43.3 ± 3.0 | 24.3 ± 3.2 | 80.0 ± 2.0 | 60.6 ± 3.2 | 32.3 ± 3.1 | 72.3 ± 6.7 | 58.7 ± 9.1 | 32.0 ± 4.0 |
CD3 and CD4 counts in hepatocyte, splenocyte or islet group were up-regulated 7 d after islet transplantation. CD8 counts in splenocyte or islet group was higher than that before islet transplantation (P < 0.05). Fourteen days after transplantation, CD3 and CD4 in hepatocyte and islet groups increased (P < 0.05), and CD8 in splenocyte and islet groups became higher than that before transplantation (P < 0.05).
| Group | Pretransplantation | 7 d post-transplantation | 14 d post-transplantation |
| Hepatocyte group | 0.34 ± 0.06 | 0.44 ± 0.02 | 0.63 ± 0.07 |
| Splenocyte group | 0.34 ± 0.06 | 0.55 ± 0.02 | 0.63 ± 0.08 |
| Mixture group | 0.34 ± 0.06 | 0.55 ± 0.01 | 0.50 ± 0.06 |
| Islet group | 0.34 ± 0.06 | 0.74 ± 0.11 | 0.81 ± 0.13 |
Table 5 shows that antibody forming function of all four kinds of islet transplantation group was significantly increased as compared with that before islets transplantation 7 or 14 d after transplantation (P < 0.05).
In islet transplantation group, there were a lot of lymphocyte infiltration, disruption of islets structure, or denaturation and necrosis of islet cells. Hepatocyte group with moderate lymphocyte infiltration. The mixture group had light lymphocytes infiltration.
Insulin dependent diabetes mellitus was considered as an autoimmune disease against beta cells[18], which is usually treated with a low dose insulin injection and immunosuppressive agents. But a large quantity of clinical data indicated that long-term insulin injection could induce insulin resistance and could not prevent some serious complications[1]. Recent studies focused on islet transplantation as a primary therapy for insulin dependent mellitus diabetes, accompanied by induction of immune tolerance to immunosuppressive agents[19,20]. Islet allo-transplantation could correct glucose imbalance and related complications, because successful islet transplantation could not only supplies the natural insulins but also other necessary biological factors. But most patients with insulin dependent diabetes mellitus could not be treated by this kind of islet transplantation because the source of allo-islets was limited. However, xenotransplantation, for example pig islet, could resolve the above-mentioned problems. Although xenotransplantation could renew the balance of glucose, rejection usually occurs, thus reducing the effect of transplantation. Islet transplantation combined with other organs or cells from the same donor could reduce rejection. Some laboratories have tried pancreas allotransplantation in combination with the same donor spleen transplantation. The results showed that the survival time of pancreas was significantly prolonged, which was attributed to the induced immunotolerance. It has been reported that bone marrow transplantation could induce the formation of hematopoiesis chimera, which could prevent acute or chronic rejection[13-15,21,22]. So it is possible to use hematopoiesis chimera to treat insulin dependent diabetes mellitus.
T lymphocyte vaccine (TCV) could induce immunotolerance for transplantation[23]. For example, TCV with recipient spleen cells pre-sensitized by donor antigen was used in rat heart transplantation, to observe the survival time of rat heart graft. The results showed that TCV could prolong the survival time of rat heart graft[24]. As to the immune response, T lymphocyte proliferation reaction increased, B lymphocyte proliferation reaction was not affected, but mixed lymphocyte reaction (MLR) declined. The analysis of phenotype showed that CD8 subpopulation increased, however there was no obvious change of antibody-dependent cell-mediated cytotoxity (ADCC) reaction. Many other studies demonstrated that liver cells (or hepatocytes) and/or spleen cells (or splenocytes) could induce specific immune tolerance in different organ transplantations, such as kidney, marrow, heart, skin[25-28]. In the aspect of islet transplantation, some scholars administrated pre-injection of donor hepatocytes and/or splenocytes to induce the immune tolerance and got the positive result [29-31].
In the present study, islet xenotransplantation was performed after pre-injection of donor hepatocytes, splenocytes, or their mixture. The results showed that the survival time of hepatocyte group, splenocyte group, mixture group was longer than islet group (P < 0.05). Pre-injection of hepatocytes, splenocytes or their mixture from the same donor was effective in preventing rejection and prolonging survival time. In the aspect of macrophage function, macrophage phagocytosis percent in islet transplantation group was increased, and the phagocytosis index in islet or hepatocyte group was increased (P < 0.05). There was no significant variation of phagocytosis percent or index in other transplantation groups 7 d after transplantation. After 14 d, phagocytosis in hepatocyte, splenocyte and islet group was significantly increased, phagocytosis index in islet or hepatocyte group was increased. The antibody-forming function of spleen B lymphocytes of recipients in hepatocyte group, splenocyte group, mixture group or islet group was increased 7 d after pig islet xenotransplantation. After 14 d, the B lymphocyte function in hepatocyte group or islet group was still higher than that before transplantation (P < 0.05). As to NK cells, NK killing activity of each experimental group, as compared with before transplantation, was increased 7 d after islet transplantation, and their NK activity maintained higher than that before transplantation 14 d after transplantation. As to the mixture group, there was no significant increase in NK activity 7 or 14 d after islet transplantation as compared with that before islet transplantation (P > 0.05). T lymphocyte subpopulation was also analyzed, the results showed that CD3 and CD4 percent of hepatocyte, splenocyte or islet group increased 7 d after islet transplantation. CD8 percent of splenocyte or islet group was higher than that before islet transplantation. Fourteen days after transplantation, CD3 and CD4 percent of hepatocyte and islet group was still higher, and CD8 percent of splenocyte and islet groups was higher than that before transplantation. To further examine immune tolerance in different experimental groups, pathological examination was performed. In islet transplantation group, lymphocyte infiltration was extensive, disruption of islet structure and necrosis of islet cells were also obvious. Hepatocyte group had moderate lymphocyte infiltration. The mixture group has slight lymphocyte infiltration. The results indicate that pre-injection of hepatocytes, splenocytes or mixture of them could reduce rejection by inducing immunotolerance. Although the concrete mechanism is not completely clear, pre-injection of hepatocytes and splenocytes from the same donor could induce immunotolerance of islet xenotransplantation, and prolong the survival time of islets.
Edited by Wang XL Proofread by Xu FM
| 1. | Oluwole OO, Depaz HA, Gopinathan R, Ali A, Garrovillo M, Jin MX, Hardy MA, Oluwole SF. Indirect allorecognition in acquired thymic tolerance: induction of donor-specific permanent acceptance of rat islets by adoptive transfer of allopeptide-pulsed host myeloid and thymic dendritic cells. Diabetes. 2001;50:1546-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Contreras JL, Eckhoff DE, Cartner S, Bilbao G, Ricordi C, Neville DM, Thomas FT, Thomas JM. Long-term functional islet mass and metabolic function after xenoislet transplantation in primates. Transplantation. 2000;69:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC. Posttransplantation diabetes: a systematic review of the literature. Diabetes Care. 2002;25:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 403] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | White SA, James RF, Swift SM, Kimber RM, Nicholson ML. Human islet cell transplantation--future prospects. Diabet Med. 2001;18:78-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Pileggi A, Ricordi C, Alessiani M, Inverardi L. Factors influencing Islet of Langerhans graft function and monitoring. Clin Chim Acta. 2001;310:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Horton PJ, Hawthorne WJ, Walters SN, Patel AT, O'Connell PJ, Chapman JR, Allen RD. Induction of allogeneic islet tolerance in a large-animal model. Cell Transplant. 2000;9:877-887. [PubMed] |
| 7. | Kahl A, Bechstein WO, Frei U. Trends and perspectives in pancreas and simultaneous pancreas and kidney transplantation. Curr Opin Urol. 2001;11:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Deng YM, Tuch BE, Rawlinson WD. Transmission of porcine endogenous retroviruses in severe combined immunodeficient mice xenotransplanted with fetal porcine pancreatic cells. Transplantation. 2000;70:1010-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Wennberg L, Song Z, Bennet W, Zhang J, Nava S, Sundberg B, Bari S, Groth CG, Korsgren O. Diabetic rats transplanted with adult porcine islets and immunosuppressed with cyclosporine A, mycophenolate mofetil, and leflunomide remain normoglycemic for up to 100 d. Transplantation. 2001;71:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Li H, Ricordi C, Inverardi L. Effects of graft-versus-host reaction on intrahepatic islet transplants. Diabetes. 1999;48:2292-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Ikebukuro K, Adachi Y, Yamada Y, Fujimoto S, Seino Y, Oyaizu H, Hioki K, Ikehara S. Treatment of streptozotocin-induced diabetes mellitus by transplantation of islet cells plus bone marrow cells via portal vein in rats. Transplantation. 2002;73:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Kawai T, Sogawa H, Koulmanda M, Smith RN, O'Neil JJ, Wee SL, Boskovic S, Sykes M, Colvin RB, Sachs DH. Long-term islet allograft function in the absence of chronic immunosuppression: a case report of a nonhuman primate previously made tolerant to a renal allograft from the same donor. Transplantation. 2001;72:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Wu T, Levay YB, Heuss N, Sozen H, Kirchhof N, Sutherland DER, Hering B, Guo Z. Inducing tolerance to MHC-matched al-logeneic islet grafts in diabetic NOD mice by simultaneous islet and bone marrow transplantation under nonirradiative and nonmyeloablative conditioning therapy. Transplantation. 2002;74:22-27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Girman P, Kríz J, Dovolilová E, Cíhalová E, Saudek F. The effect of bone marrow transplantation on survival of allogeneic pancreatic islets with short-term tacrolimus conditioning in rats. Ann Transplant. 2001;6:43-45. [PubMed] |
| 15. | Maria-Engler SS, Mares-Guia M, Correa ML, Oliveira EM, Aita CA, Krogh K, Genzini T, Miranda MP, Ribeiro M, Vilela L. Microencapsulation and tissue engineering as an alternative treatment of diabetes. Braz J Med Biol Res. 2001;34:691-697. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Gamian E, Kochman A, Rabczynski J, Burczak K. Biocompati-bility testing and function of a pancreatic prosthesis consisting of viable pancreatic islets encapsulated in PVA macrocapsules. Polim Med. 1999;29:3-20. |
| 17. | Heiser A, Ulrichs K, Müller-Ruchholtz W. Isolation of porcine pancreatic islets: low trypsin activity during the isolation procedure guarantees reproducible high islet yields. J Clin Lab Anal. 1994;8:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Petruzzo P, Andreelli F, McGregor B, Lefrançois N, Dawahra M, Feitosa LC, Dubernard JM, Thivolet C, Martin X. Evidence of recurrent type I diabetes following HLA-mismatched pancreas transplantation. Diabetes Metab. 2000;26:215-218. [PubMed] |
| 19. | Thomas JM, Contreras JL, Smyth CA, Lobashevsky A, Jenkins S, Hubbard WJ, Eckhoff DE, Stavrou S, Neville DM Jr, Thomas FT. Successful reversal of streptozotocin-induced diabetes with stable allogeneic islet function in a preclinical model of type 1 diabetes. Diabetes. 2001;50:1227-1236. [RCA] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Sutherland DE, Gruessner RW, Dunn DL, Matas AJ, Humar A, Kandaswamy R, Mauer SM, Kennedy WR, Goetz FC, Robertson RP. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233:463-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 427] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 21. | Good RA, Verjee T. Historical and current perspectives on bone marrow transplantation for prevention and treatment of immunodeficiencies and autoimmunities. Biol Blood Marrow Transplant. 2001;7:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Ciancio G, Miller J, Garcia-Morales RO, Carreno M, Burke GW, Roth D, Kupin W, Tzakis AG, Ricordi C, Rosen A. Six-year clinical effect of donor bone marrow infusions in renal transplant patients. Transplantation. 2001;71:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Lakey JR, Singh B, Warnock GL, Elliott JF, Rajotte RV. Long-term survival of syngeneic islet grafts in BCG-treated diabetic NOD mice can be reversed by cyclophosphamide. Transplantation. 1995;59:1751-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Shanqi Y, Suisheng X. Study on the mechanisms of T cell vacci-nation-induced survival prolongation of cardiac allograft in rats. Zhonghua Qiguan Yizhi Zazhi. 2000;21:303-305. |
| 25. | Motoyama K, Arima T, Yu S, Lehmann M, Flye MW. The kinetics of tolerance induction by nondepleting anti-CD4 monoclonal antibody (RIB 5/2) plus intravenous donor alloantigen administration. Transplantation. 2000;69:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Smyk-Pearson SK, Bakke AC, Held PK, Wildin RS. Rescue of the autoimmune scurfy mouse by partial bone marrow transplantation or by injection with T-enriched splenocytes. Clin Exp Immunol. 2003;133:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Nakafusa Y, Goss JA, Mohanakumar T, Flye MW. Induction of donor-specific tolerance to cardiac but not skin or renal allografts by intrathymic injection of splenocyte alloantigen. Transplantation. 1993;55:877-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Dono K, Maki T, Wood ML, Monaco AP. Induction of tolerance to skin allografts by intrathymic injection of donor splenocytes. Effect of donor-recipient strain combination and supplemental rapamycin. Transplantation. 1995;60:1268-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Sun J, Wang X, Wang C, Sheil AG. Sequential transplantation induces islet allograft tolerance. Microsurgery. 2001;21:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Sutherland DE, Gruessner RW, Gruessner AC. Pancreas transplantation for treatment of diabetes mellitus. World J Surg. 2001;25:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Sakuma Y, Uchida H, Nagai H, Kobayashi E. High-dose tacrolimus and lengthy survival of the combined rat pancreas/spleen graft in a high-responder combination. Transpl Immunol. 2001;9:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |