Published online Jan 1, 2004. doi: 10.3748/wjg.v10.i1.22
Revised: July 20, 2003
Accepted: August 16, 2003
Published online: January 1, 2004
AIM: To study the effect of NF-κB, survivin, Bcl-2 and Caspase3 on tumor necrosis factors related apoptosis inducing ligand (TRAIL) induced apoptosis of gastric cancer cells.
METHODS: Gastric cancer cells of SGC-7901, MKN28, MKN45 and AGS lines were cultured in PRMI-1640 medium and the apoptosis rates of the cells of 4 lines were observed after treatment of tumor necrosis factors related apoptosis inducing ligand (TRAIL) with a flow cytometer. The expression of NF-κB, survivin, Bcl-2 and Caspase3 in gastric cancer cells of 4 lines was analyzed with Western blot.
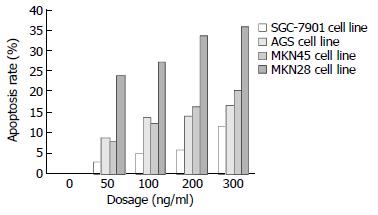
RESULTS: After the gastric cancer cells were exposed to TRAIL 300 ng/mL for 24 hours, the apoptosis rate was 36.05%, 20.27%, 16.50% and 11.80% in MKN28, MKN45, AGS and SGC-7901cells respectively. Western blot revealed that the expressions of NF-κB and survivin were lower in MKN28 cells than in MKN45, AGS and SGC-7901 cells. In contrast, the expression of Caspase3 was higher in MKN28 cells than in MKN45, AGS and SGC-7901 cells.
CONCLUSION: There is a selectivity of TRAIL potency to induce apoptosis in gastric cancer cells of different cell lines. The anticancer potency of TRAIL is associated with the decreased expression of NF-κB and survivin and increased expression of Caspase3 of gastric cancer cells.
- Citation: Yang LQ, Fang DC, Wang RQ, Yang SM. Effect of NF-κB, survivin, Bcl-2 and Caspase3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand. World J Gastroenterol 2004; 10(1): 22-25
- URL: https://www.wjgnet.com/1007-9327/full/v10/i1/22.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i1.22
Tumor necrosis factors related apoptosis inducing ligand (TRAIL) is one of the members of TNF family. TRAIL, also known as APO-2L[1,2], is highly homologous to FasL. TRAIL is capable of inducing apoptosis of many kinds of cancer cells but nontoxic to normal cells[3-13]. It has been found that cells of different cancers and even different types of cells of a cancer exhibit significantly different sensitivity to TRAIL[14-17].
Nuclear transcript factor (NF-κB), survivin and Bcl-2 are apoptosis inhibitors and play a key role in the mechanism of anti-apoptosis of tumors[18-29]. If the activity of these factors is suppressed, tumor cells can undergo apoptosis and stop growing[30,31]. Various modulation elements of cell apoptosis exert their action through Caspase enzyme system. Among them, Caspase 3 (also known as CPP32, YAMA, or apopain) is probably the one that so far best correlates with apoptosis[32,33]. Furthermore, Caspase 3 expression could be detected in several human malignancies such as non-small cell lung carcinoma[34], esophageal squamous cell carcinoma[35] and gastric cancer[36]. The inhibition of cell apoptosis is closely related to the onset, development and sensitivity to chemotherapy of malignant tumors. Consequently, we studied the relationship of the sensitivity of the cells of 4 gastric cancer cell lines to TRAIL with the expression of NF-κB, survivin, Bcl-2 and Caspase3 in order to clarify why and how gastric cancer cells were resistant to TRAIL.
SGC-7901 line of gastric cancer cells was preserved in our laboratory. MKN28, MKN45 and AGS cell lines were donated by our colleagues of the Fourth Military Medical University. NC membrane and mouse antihuman survivin monoclonal antibody were bought from Santa Cruz Biotech Company. Mouse antihuman NF-κB (p65) monoclonal antibody, mouse antihuman Bcl-2 monoclonal antibody, mouse antihuman Caspase3 monoclonal antibody and peroxidase-conjugated rabbit anti-mouse IgG were from the Zhongshan Company in Beijing, and ECL chemofluerescent agent kit was from DingguoBiotech Center in Beijing. TRAIL protein was prepared by ourselves[37].
Gastric cancer cells of SGC-7901, MKN28, MKN45 and AGS lines were incubated in PRMI-1640 medium and 10% inactivated calf serum. Penicillin and streptomycin were added to a final concentration of 100 u/mL. Generation transition of the cells was achieved every 3 to 5 days through the adoption of 0.25% pancreatic enzyme and 0.02% EDTA.
Gastric cancer cells in the logarithmic proliferation stage were studied. The culture medium of every cell line was distubated into 5 bottles (100 mL in each). TRAIL was added to the 5 bottles of every cell line with a final concentration of 0 ng/mL, 50 ng/mL, 100 ng/mL, 200 ng/mL and 300 ng/mL respectively. All the bottles were incubated under 37 °C, 5% CO2 and saturated humidity for 24 hours.
The apoptosis rate of gastric cancer cells was determined with a flow cytometer. All the cells were collected, digested and washed twice in PBS, and the floating cells were precipitated with 0.1 mL PBS. After the cells were fixed in 70% precooled ethanol for 24 hours, they were stained with PI and examined.
Western blot was used to determine the expression of NF-κB, survivin, Bcl-2 and Caspase3. The gastric cancer cells wereruptured with lysozyme and protein concentration of cells was measured with Lowry method. The protein concentration was unified with PBS. In order to denature the protein, the samples were heated with middle molecular weight protein Marker to 100 °C for 3 minutes. The protein, after being separated with SDS-PAGE electrophoresis, was transferred to a piece of nitrocellulose membrane with moisture transfer technique at 100v for 2 hours. The membrane was stained with ponceau-S to confirm whether the protein transfer was successful. Five percent of defat milk powder-PBS solution was used to block the unspecific antibody binding site for one hour. Then the membrane was washed 3 times in PBS-T solution, 15 minutes each time. Monoclonal antibody solution (1:1 000) of mouse antihuman NF-κB, survivin, Bcl-2 and Caspase3 was incubated with the membrane for 1 hour respectively. Again, each piece of membrane was washed 3 times in PBS-T solution, 15 minutes each time. Then, all the membranes were incubated with rabbit antimouse IgG solution (1:1 000) for one hour. The membranes were washed 3 times in PBS-T solution, 15 minutes each time. Eventually, the membranes were incubated with chemofluorescent agent for 5 minutes, and then were exposed to X-ray films in a dark room. The X-ray films were developed and examined.
SPSS statistic soft package was used to undergo single factor Chi square analysis and t test. P < 0.05 was considered as significant.
TRAIL exerted a considerable apoptosis-inducing effect on the 4 line gastric cancer cells and the effect was in a dosage dependent manner. Figure 1 shows that the gastric cancer cells of the 4 lines had a different sensitivity to TRAIL. After the action of 300 ng/mL TRAIL for 24 hours, the apoptosis rate was 36.05% in MKN28, 20.27% in MKN45, 16.50% in AGS and 11.80% in SGC-7901 lines. The apoptosis rate was significantly higher in MKN28 than in other 3 kinds of cells (P < 0.05), but there was no significant difference among the other 3 lines of gastric cancer cells. SGC-7901 cells were resistant to TRAIL.
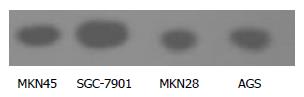
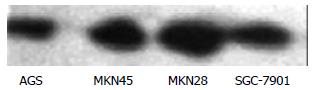
The expression of NF-κB (p65) in the 4 lines of gastric cancer cells is shown in Figure 2. NF-κB expression was lower in MKN28 than in SGC-7901 cells, but no significant difference was found between MKN45 and AGS cells.
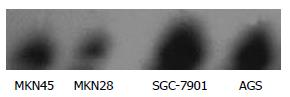
Before administration of TRAIL, Western blot revealed that the expression of survivin was significantly lower in MKN28 than in SGC-7901 cells, and no significant difference was found between MKN45 and AGS cells (Figure 3).
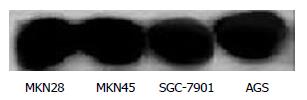
Before administration of TRAIL, Western blot revealed that the expression of Bcl-2 had no significant difference in the 4 line gastric cancer cells (Figure 4).
Before administration of TRAIL, Western blot showed that the expression of Caspase 3 was lower in SGC-7901 and highest in MKN28 cells. The expression in AGS and MKN45 cells was in the middle range (Figure 5).
TRAIL is a recently discovered apoptosis inducing molecule. It has aroused great interest in the medical circle because it can selectively induce cancer cells, transform cells and virus-infected cells to undergo apoptosis with no toxicity to normal cells. In this study, anticancer effect of TRAIL on the 4 lines of gastric cancer cells was observed. We found that TRAIL exerted a certain degree of apoptosis inducing action on the gastric cancer cells and the action potency was dosage dependent. Twenty-four hours after administration of 300 ng/mL TRAIL, the apoptosis rate was 36.05% in MKN28, 20.27% in MKN45, 16.50% in AGS and 11.80% in SGC-7901 cells. This implies that the sensitivity to apoptosis inducing action of TRAIL varied among the 4 lines of gastric cancer cells.
Apoptosis is a cellular suicidal action. This process is under the combined modulation of apoptosis promoting factors including p53, Fas and others and apoptosis inhibiting factors including Bcl-2, CIAP, survivin and others. Survivin is an inhibitor of apoptosis protein (IAP), which has been found to be crucial for mitosis and cell cycle progression[38]. Survivin could act directly on Caspases and mainly suppress the activity of Caspase 3 and Caspase7[39]. Disruption of survivin-microtubule interactions could result in loss of survivin’s anti-apoptosis function and increase caspase-3 activity, a mechanism involved in cell death during mitosis[40]. In addition, it has been found that deficiency of survivin in transgenic mice could exacerbate Fas-induced apoptosis via mitochondrial pathways[41]. In the present study, we found that the difference of sensitivity to TRAIL among the 4 lines of gastric cancer cells was related to the expression of survivin and Caspase of the cells. SGC-7901 cells with high expression of survivin and low expression of Caspase 3 were resistant to TRAIL, but MKN28 cells with low expression of survivin and high expression of Caspase 3 were sensitive to TRAIL. So, the determination of the expression level of survivin and Caspase 3 in gastric cancer cells is helpful to determine whether the cells are sensitive to TRAIL. To suppress the expression of survivin and increase the expression of Caspase 3 could strengthen the sensitivity of gastric cancer cells to TRAIL[42].
The transcription factor NF-κB is a key regulator of immune responses and inflammation operating through the induction of numerous genes, including those coding for cytokines, chemokines and adhesion molecules[43-45]. NF-κB suppresses apoptosis by inducing expression of a number of genes whose products inhibit apoptosis, including inhibitors of apoptosis (IAPs), Caspase 8-FADD-like IL-1β-converting enzyme (Caspase 8-FLICE) inhibitory protein (cFLIP), A1 (also known as Bfl1), TNF receptor associated factor 1 (TRAF1) and TRAF2. These anti-apoptotic proteins have been found to work in a coordinated fashion to block apoptosis at multiple steps along the apoptotic cascade or to regulate other pro- or anti-apoptotic pathways[46]. NF-κB could also regulate the expression of several members of the Bcl-2 family[47]. In this study, we found that the sensitivity of the 4 line gastric cancer cells to TRAIL was related to the expression of NF-κB. SGC-7901 cells showed a relatively high expression of NF-κB and were resistant to TRAIL. On the contrary, MKN28 cells with a low expression of NF-κB were sensitive to TRAIL. It is considered that suppression of the expression of NF-κB in gastric cancer cells can increase their sensitivity to TRAIL.
Bcl-2 is a proto-oncogene and can suppress apoptosis. Bcl-2 has been found to be closely related to the onset and drug resistance of many kinds of malignant tumors[48-52]. Previous studies have confirmed that overexpression of Bcl-2 in the cells of various malignant tumors could result in resistance against cell apoptosis induced by chemotherapeutic agents such as cisplatin[53,54] and arsenic trioxide[55]. If Bcl-2 expression is suppressed, tumors are impelled to undergo apoptosis. We found that the sensitivity difference among the 4 lines of gastric cancer cells was not related with Bcl-2 expression, but the sensitivity difference to TRAIL did occur among them. This finding was in agreement with the recently published data on breast cancer[56].
To sum up, TRAIL possesses the characteristics of selectively inducing apoptosis of tumor cells. TRAIL-resistant tumor cells may be related to the expression of apoptosis-inhibitor survivin and NF-κB. Suppression of the expression of survivin and NF-κB, and reinforcement of Caspase 3 expression would increase the sensitivity of gastric cancer cells to TRAIL. Further study is imperative to clarify the mechanism of the apoptosis-inducing action of TRAIL.
Edited by Xu JY and Wang XL
| 1. | Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687-12690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1354] [Cited by in RCA: 1381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 2. | LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 653] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 3. | Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1924] [Cited by in RCA: 1927] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 4. | Silvestris F, Cafforio P, Tucci M, Dammacco F. Negative regulation of erythroblast maturation by Fas-L(+)/TRAIL(+) highly malignant plasma cells: a major pathogenetic mechanism of anemia in multiple myeloma. Blood. 2002;99:1305-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Odoux C, Albers A, Amoscato AA, Lotze MT, Wong MK. TRAIL, FasL and a blocking anti-DR5 antibody augment paclitaxel-induced apoptosis in human non-small-cell lung cancer. Int J Cancer. 2002;97:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Seki N, Hayakawa Y, Brooks AD, Wine J, Wiltrout RH, Yagita H, Tanner JE, Smyth MJ, Sayers TJ. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res. 2003;63:207-213. [PubMed] |
| 7. | Kim DM, Koo SY, Jeon K, Kim MH, Lee J, Hong CY, Jeong S. Rapid induction of apoptosis by combination of flavopiridol and tumor necrosis factor (TNF)-alpha or TNF-related apoptosis-inducing ligand in human cancer cell lines. Cancer Res. 2003;63:621-626. [PubMed] |
| 8. | Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MR, Yagita H. Nature's TRAIL--on a path to cancer immunotherapy. Immunity. 2003;18:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 250] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. |
Guillermet J, Saint-Laurent N, Rochaix P, Cuvillier O, Levade T, Schally AV, Pradayrol L, Buscail L, Susini C, Bousquet C. Somatostatin receptor subtype 2 sensitizes human pancreatic cancer cells to death ligand-induced apoptosis.
Proc Natl Acad Sci |
| 10. | Naka T, Sugamura K, Hylander BL, Widmer MB, Rustum YM, Repasky EA. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients' colon tumors grown in SCID mice. Cancer Res. 2002;62:5800-5806. [PubMed] |
| 11. | Wajant H, Pfizenmaier K, Scheurich P. TNF-related apoptosis inducing ligand (TRAIL) and its receptors in tumor surveillance and cancer therapy. Apoptosis. 2002;7:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Inoue H, Shiraki K, Yamanaka T, Ohmori S, Sakai T, Deguchi M, Okano H, Murata K, Sugimoto K, Nakano T. Functional expression of tumor necrosis factor-related apoptosis-inducing ligand in human colonic adenocarcinoma cells. Lab Invest. 2002;82:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Wei XC, Wang XJ, Chen K, Zhang L, Liang Y, Lin XL. Killing effect of TNF-related apoptosis inducing ligand regulated by tetracycline on gastric cancer cell line NCI-N87. World J Gastroenterol. 2001;7:559-562. [PubMed] |
| 14. | MacFarlane M, Harper N, Snowden RT, Dyer MJ, Barnett GA, Pringle JH, Cohen GM. Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lymphocytic leukaemia. Oncogene. 2002;21:6809-6818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Held J, Schulze-Osthoff K. Potential and caveats of TRAIL in cancer therapy. Drug Resist Updat. 2001;4:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Ruiz de Almodóvar C, Ruiz-Ruiz C, Muñoz-Pinedo C, Robledo G, López-Rivas A. The differential sensitivity of Bc1-2-overexpressing human breast tumor cells to TRAIL or doxorubicin-induced apoptosis is dependent on Bc1-2 protein levels. Oncogene. 2001;20:7128-7133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Ibrahim SM, Ringel J, Schmidt C, Ringel B, Müller P, Koczan D, Thiesen HJ, Löhr M. Pancreatic adenocarcinoma cell lines show variable susceptibility to TRAIL-mediated cell death. Pancreas. 2001;23:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Ohshima K, Sugihara M, Haraoka S, Suzumiya J, Kanda M, Kawasaki C, Shimazaki K, Kikuchi M. Possible immortalization of Hodgkin and Reed-Sternberg cells: telomerase expression, lengthening of telomere, and inhibition of apoptosis by NF-kappaB expression. Leuk Lymphoma. 2001;41:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Wall NR, O'Connor DS, Plescia J, Pommier Y, Altieri DC. Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer Res. 2003;63:230-235. [PubMed] |
| 20. | Hussein MR, Haemel AK, Wood GS. Apoptosis and melanoma: molecular mechanisms. J Pathol. 2003;199:275-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Kim IK, Jung YK, Noh DY, Song YS, Choi CH, Oh BH, Masuda ES, Jung YK. Functional screening of genes suppressing TRAIL-induced apoptosis: distinct inhibitory activities of Bcl-XL and Bcl-2. Br J Cancer. 2003;88:910-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21:2283-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 304] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 23. | Zhou HB, Zhu JR. Paclitaxel induces apoptosis in human gastric carcinoma cells. World J Gastroenterol. 2003;9:442-445. [PubMed] |
| 24. | Guo XZ, Shao XD, Liu MP, Xu JH, Ren LN, Zhao JJ, Li HY, Wang D. Effect of bax, bcl-2 and bcl-xL on regulating apoptosis in tissues of normal liver and hepatocellular carcinoma. World J Gastroenterol. 2002;8:1059-1062. [PubMed] |
| 25. | Li HL, Chen DD, Li XH, Zhang HW, Lu YQ, Ye CL, Ren XD. Changes of NF-kB, p53, Bcl-2 and caspase in apoptosis induced by JTE-522 in human gastric adenocarcinoma cell line AGS cells: role of reactive oxygen species. World J Gastroenterol. 2002;8:431-435. [PubMed] |
| 26. | Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403-406. [PubMed] |
| 27. | Wang LD, Zhou Q, Wei JP, Yang WC, Zhao X, Wang LX, Zou JX, Gao SS, Li YX, Yang C. Apoptosis and its relationship with cell proliferation, p53, Waf1p21, bcl-2 and c-myc in esophageal carcinogenesis studied with a high-risk population in northern China. World J Gastroenterol. 1998;4:287-293. [PubMed] |
| 28. | Liu HF, Liu WW, Fang DC, Men RP. Expression of bcl-2 protein in gastric carcinoma and its significance. World J Gastroenterol. 1998;4:228-230. [PubMed] |
| 29. | Zhou HB, Yan Y, Sun YN, Zhu JR. Resveratrol induces apoptosis in human esophageal carcinoma cells. World J Gastroenterol. 2003;9:408-411. [PubMed] |
| 30. | Dalen H, Neuzil J. Alpha-tocopheryl succinate sensitises a T lymphoma cell line to TRAIL-induced apoptosis by suppressing NF-kappaB activation. Br J Cancer. 2003;88:153-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Biswas DK, Martin KJ, McAlister C, Cruz AP, Graner E, Dai SC, Pardee AB. Apoptosis caused by chemotherapeutic inhibition of nuclear factor-kappaB activation. Cancer Res. 2003;63:290-295. [PubMed] |
| 32. | Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 3157] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 33. | Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269:30761-30764. [PubMed] |
| 34. | Törmänen-Näpänkangas U, Soini Y, Kahlos K, Kinnula V, Pääkkö P. Expression of caspases-3, -6 and -8 and their relation to apoptosis in non-small cell lung carcinoma. Int J Cancer. 2001;93:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Hsia JY, Chen CY, Chen JT, Hsu CP, Shai SE, Yang SS, Chuang CY, Wang PY, Miaw J. Prognostic significance of caspase-3 expression in primary resected esophageal squamous cell carcinoma. Eur J Surg Oncol. 2003;29:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Kania J, Konturek SJ, Marlicz K, Hahn EG, Konturek PC. Expression of survivin and caspase-3 in gastric cancer. Dig Dis Sci. 2003;48:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Li XA, Fang DC, Yang SM, Luo YH. The expression, purification and anticancer activity of TRAIL. Acta Acad Med Milit Tert. 2002;23:1058-1060. |
| 38. | Suzuki A, Hayashida M, Ito T, Kawano H, Nakano T, Miura M, Akahane K, Shiraki K. Survivin initiates cell cycle entry by the competitive interaction with Cdk4/p16(INK4a) and Cdk2/cyclin E complex activation. Oncogene. 2000;19:3225-3234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 158] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 526] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 40. | Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1442] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 41. | Conway EM, Pollefeyt S, Steiner-Mosonyi M, Luo W, Devriese A, Lupu F, Bono F, Leducq N, Dol F, Schaeffer P. Deficiency of survivin in transgenic mice exacerbates Fas-induced apoptosis via mitochondrial pathways. Gastroenterology. 2002;123:619-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Wall NR, O’Connor DS, Plescia J, Pommier Y, Altieri DC. Sup-pression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer Res. 2003;63:230-235 [PMid:12517802]. |
| 43. | Gong JP, Liu CA, Wu CX, Li SW, Shi YJ, Li XH. Nuclear factor kB activity in patients with acute severe cholangitis. World J Gastroenterol. 2002;8:346-349. [PubMed] |
| 44. | Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1080] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 45. | Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl:S81-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2908] [Cited by in RCA: 2962] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 46. | Lin A, Karin M. NF-kappaB in cancer: a marked target. Semin Cancer Biol. 2003;13:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 281] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 47. | Wang CY, Guttridge DC, Mayo MW, Baldwin AS. NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923-5929. [PubMed] |
| 48. | Šošić D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 369] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 49. | Zhou HB, Zhu JR. Paclitaxel induces apoptosis in human gastric carcinoma cells. World J Gastroenterol. 2003;9:442-445 [PMid:12632493]. |
| 50. | Liu JR, Chen BQ, Yang YM, Wang XL, Xue YB, Zheng YM, Liu RH. Effect of apoptosis on gastric adenocarcinoma cell line SGC-7901 induced by cis-9, trans-11-conjugated linoleic acid. World J Gastroenterol. 2002;8:999-1004. [PubMed] |
| 51. | Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792-796. [PubMed] |
| 52. | Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796-800. [PubMed] |
| 53. | Siervo-Sassi RR, Marrangoni AM, Feng X, Naoumova N, Winans M, Edwards RP, Lokshin A. Physiological and molecular effects of Apo2L/TRAIL and cisplatin in ovarian carcinoma cell lines. Cancer Lett. 2003;190:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Rudin CM, Yang Z, Schumaker LM, VanderWeele DJ, Newkirk K, Egorin MJ, Zuhowski EG, Cullen KJ. Inhibition of glutathione synthesis reverses Bcl-2-mediated cisplatin resistance. Cancer Res. 2003;63:312-318. [PubMed] |
| 55. | Xu HY, Yang YL, Gao YY, Wu QL, Gao GQ. Effect of arsenic trioxide on human hepatoma cell line BEL-7402 cultured in vitro. World J Gastroenterol. 2000;6:681-687. [PubMed] |
| 56. | Kim IK, Jung YK, Noh DY, Song YS, Choi CH, Oh BH, Masuda ES, Jung YK. Functional screening of genes suppressing TRAIL-induced apoptosis: distinct inhibitory activities of Bcl-XL and Bcl-2. Br J Cancer. 2003;88:910-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |