Copyright
©The Author(s) 2002.
World J Gastroenterol. Feb 15, 2002; 8(1): 1-4
Published online Feb 15, 2002. doi: 10.3748/wjg.v8.i1.1
Published online Feb 15, 2002. doi: 10.3748/wjg.v8.i1.1
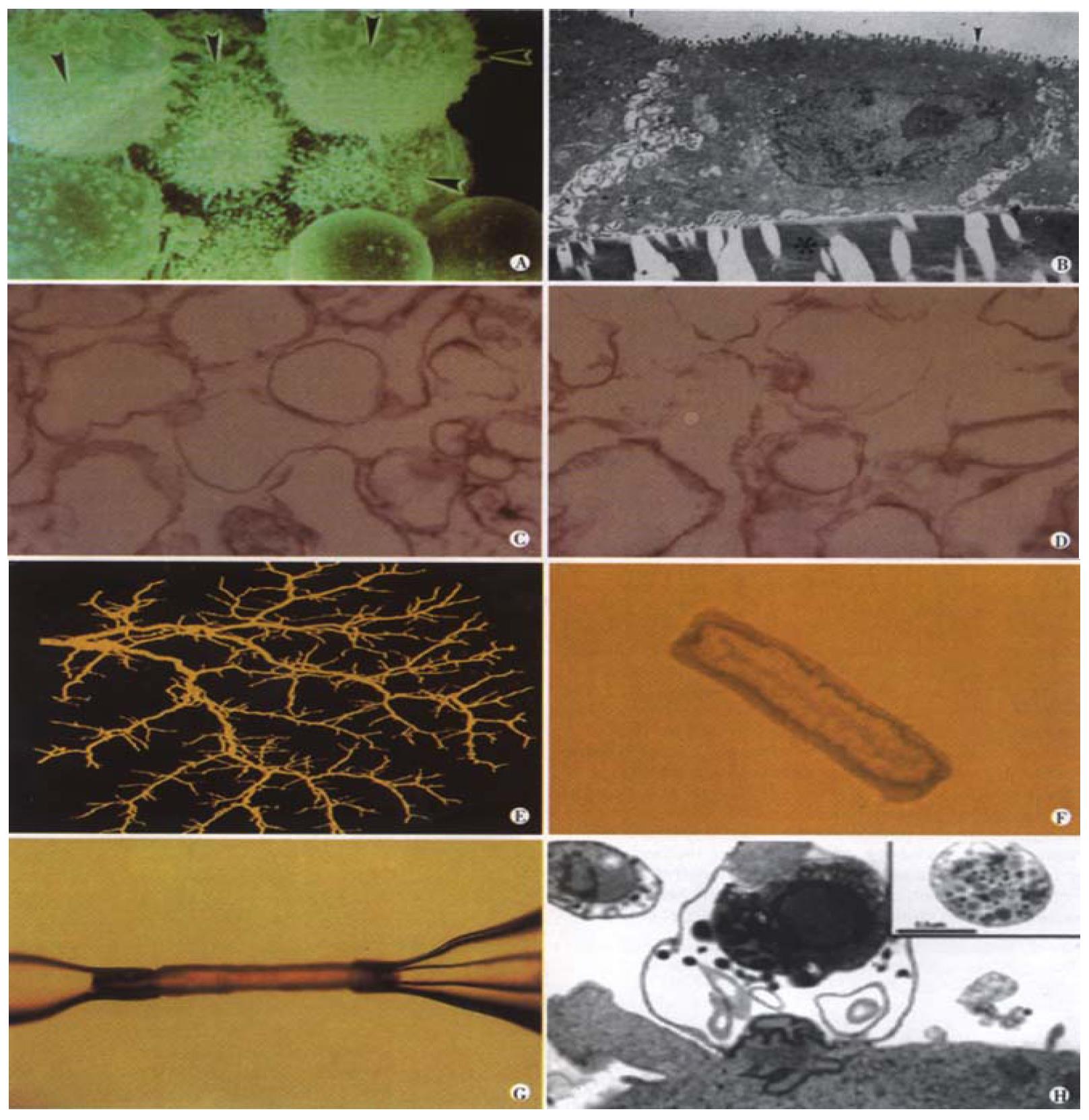
Figure 1 Experimental in vitro models of biliary epithelia.
(a) A scanning electron micrograph of a group of isolated cholangiocytes after separation using immunomagnetic beads. Note that the prominent microvilli (arrowheads) are limited to one side of the cells. Mag = 4400. (b) A transmission electron micrograph cross-section of normal rat cholangiocytes in culture demonstrates characteristics of polarized cells with apical microvilli (arrowheads) and numerous basolateral intercellular interdigitations near the collagen coated filter denoted by *, (Bar = 2 mm, Mag = 7500). (c) (d) A transmission electron micrograph of apical (c) and basolateral (d) plasma membrane domains revealed similar homogenous vesiculated membranes of varied shapes and sizes without apparent contamination of other organelles (Bar = 0.5 mm, Mag = 22500). (e) A three-dimensional reconstructed image of the intrahepatic biliary tree isolated from normal rat liver. (f) A light micrograph of an unstained isolated bile duct unit from rat liver. After overnight culture, the two ends seal forming an enclosed unit. A single layer of epithelial cells with a thin outer layer of connective tissue surrounds the lumen. (g) A microperfused intrahepatic bile duct unit isolated from rat liver, manually dissected and cannulated with micropipettes. (h) Transmission electron micrograph of C. parvum infection of cultured human cholangiocytes. A parasitophorous vacuole contains a developing parasite stage which is intracellular but extracytoplasmic. A macrogamet is shown in the inset, demonstrating development of sexual stages of the parasite. Bar = 1 mm.
- Citation: Tietz PS, Chen XM, Gong AY, Huebert RC, Masyuk A, Masyuk T, Splinter PL, LaRusso NF. Experimental models to study cholangiocyte biology. World J Gastroenterol 2002; 8(1): 1-4
- URL: https://www.wjgnet.com/1007-9327/full/v8/i1/1.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i1.1