Copyright
©The Author(s) 2022.
World J Gastroenterol. Aug 14, 2022; 28(30): 4182-4200
Published online Aug 14, 2022. doi: 10.3748/wjg.v28.i30.4182
Published online Aug 14, 2022. doi: 10.3748/wjg.v28.i30.4182
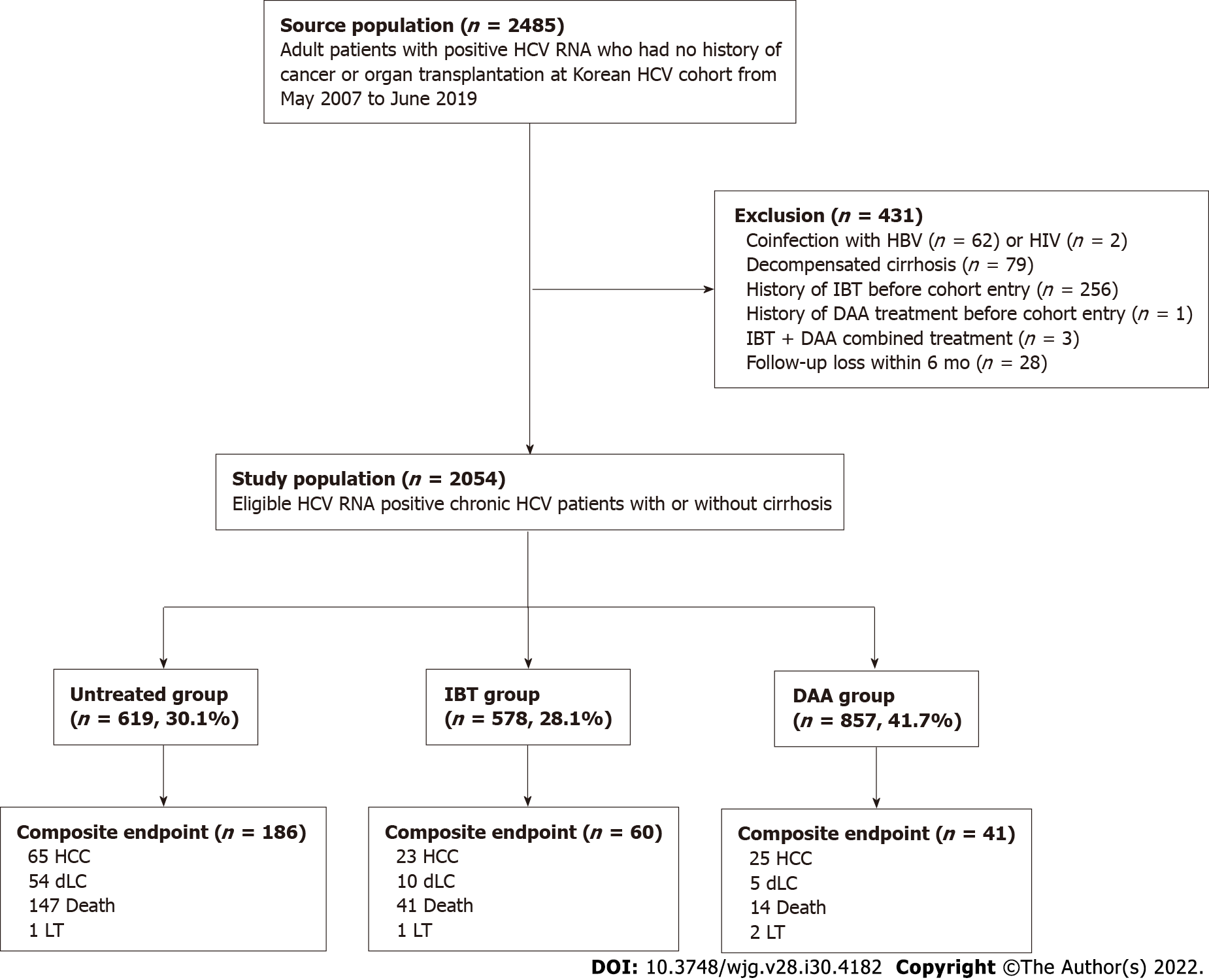
Figure 1 Patient flow diagram.
DAA: Direct-acting antivirals; dLC: Decompensated liver cirrhosis; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus; IBT: Interferon-based treatment; HIV: Human immunodeficiency virus; LT: Liver transplantation.
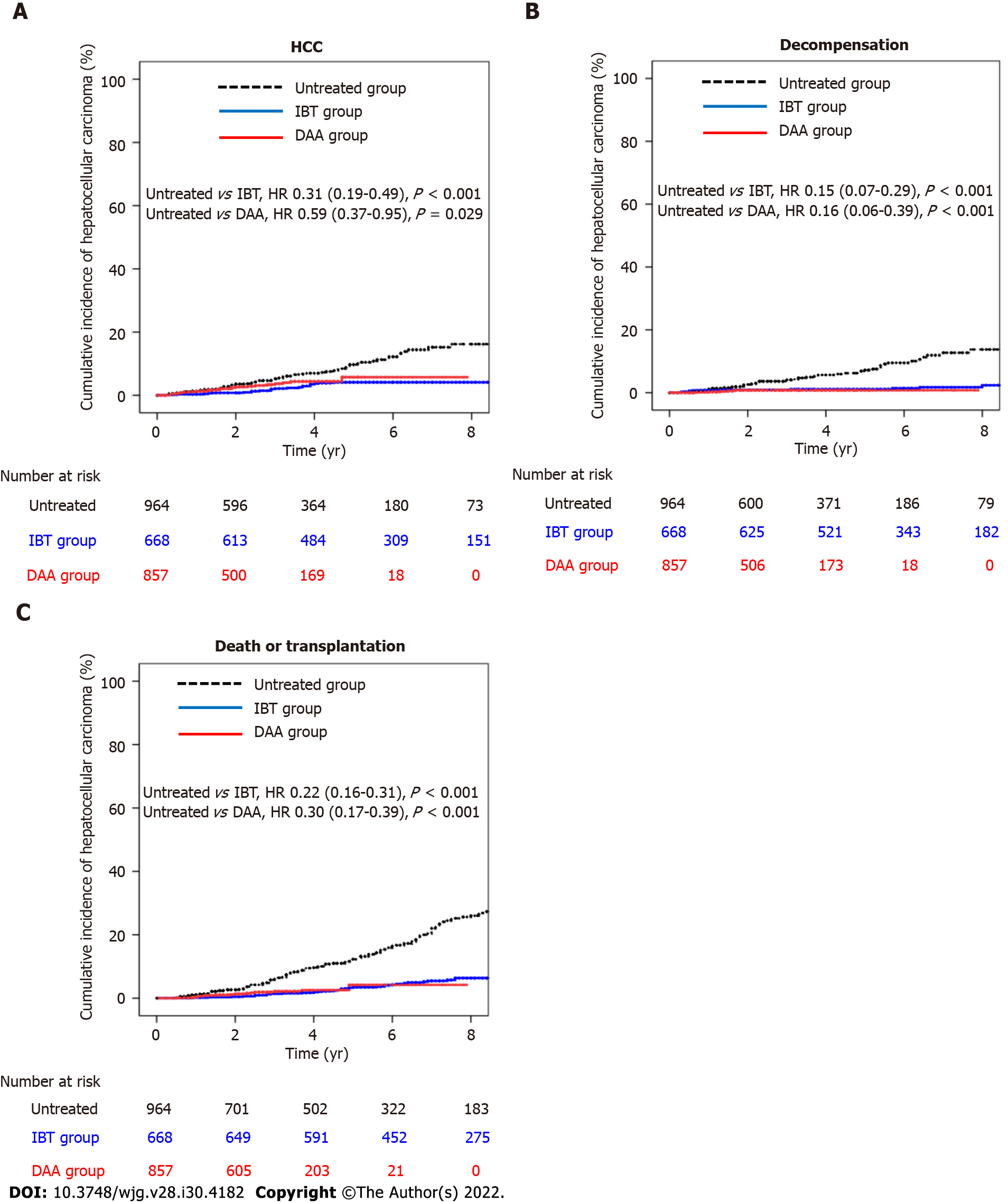
Figure 2 Cumulative incidences of hepatocellular carcinoma, decompensation, and death/transplantation in entire cohort.
A: Cumulative incidence of hepatocellular carcinoma in the untreated, interferon-based treatment (IBT), and direct-acting antivirals (DAA) groups; B: Cumulative incidence of decompensation in the untreated, IBT, and DAA groups; C: Cumulative incidence of death/transplantation in the untreated, IBT, and DAA groups. DAA: Direct-acting antivirals; HCC: Hepatocellular carcinoma; IBT: Interferon-based treatment.
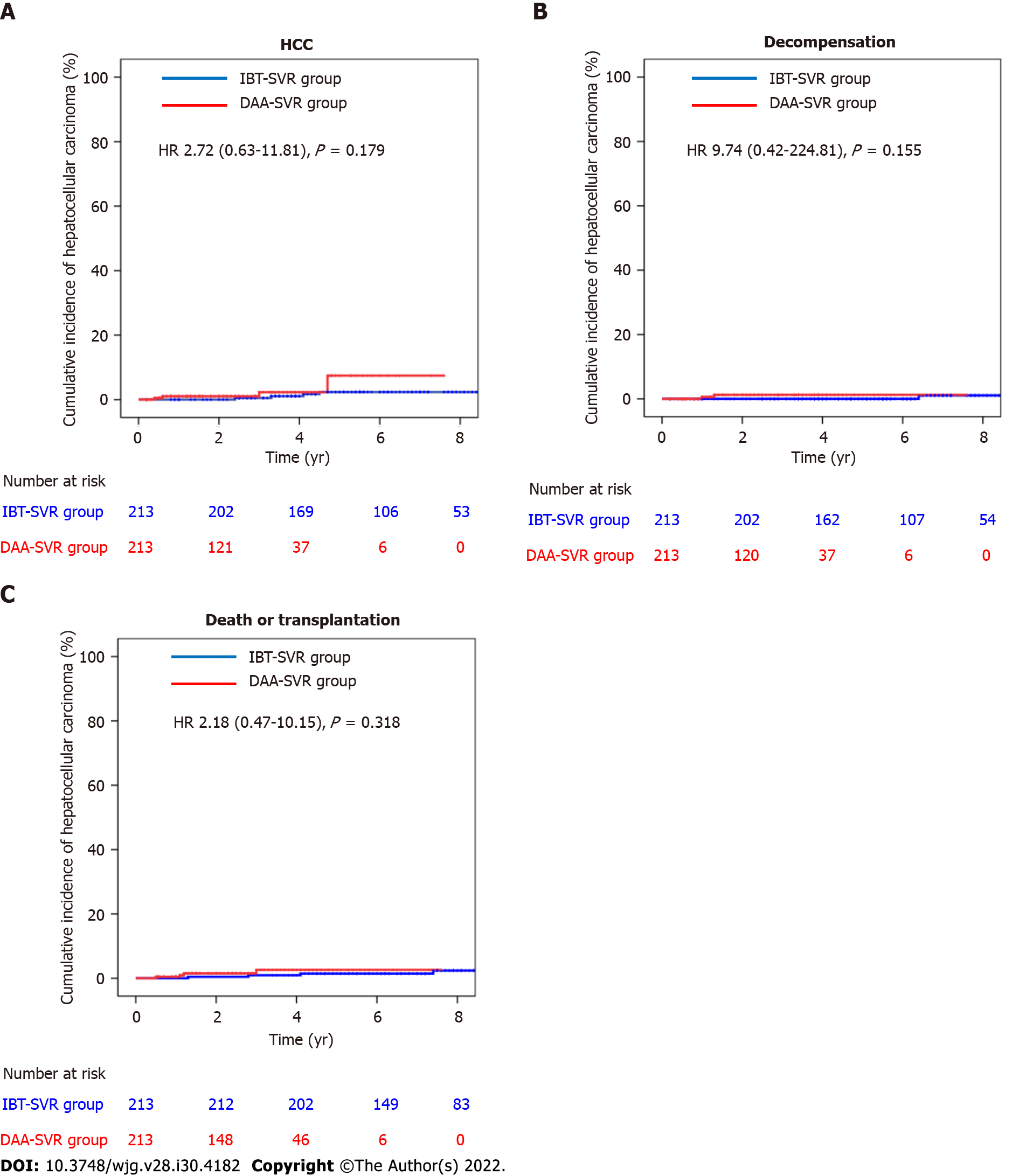
Figure 3 Cumulative incidences of hepatocellular carcinoma, decompensation, and death/transplantation in the matched sustained virologic response cohort.
A: Cumulative incidence of hepatocellular carcinoma in the interferon-based treatment-sustained virologic response (IBT-SVR) and direct-acting antivirals-sustained virologic response (DAA-SVR) groups; B: Cumulative incidence of decompensation in the IBT-SVR and DAA-SVR groups; C: Cumulative incidence of death/Liver transplantation in the IBT-SVR and DAA-SVR groups. DAA: Direct-acting antivirals; HCC: Hepatocellular carcinoma; IBT: Interferon-based treatment; SVR: Sustained virologic response.
- Citation: Choi GH, Jang ES, Kim YS, Lee YJ, Kim IH, Cho SB, Lee HC, Jang JW, Ki M, Choi HY, Baik D, Jeong SH. Hepatocellular carcinoma, decompensation, and mortality based on hepatitis C treatment: A prospective cohort study. World J Gastroenterol 2022; 28(30): 4182-4200
- URL: https://www.wjgnet.com/1007-9327/full/v28/i30/4182.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i30.4182