Copyright
©The Author(s) 2021.
World J Gastroenterol. Jan 21, 2021; 27(3): 240-254
Published online Jan 21, 2021. doi: 10.3748/wjg.v27.i3.240
Published online Jan 21, 2021. doi: 10.3748/wjg.v27.i3.240
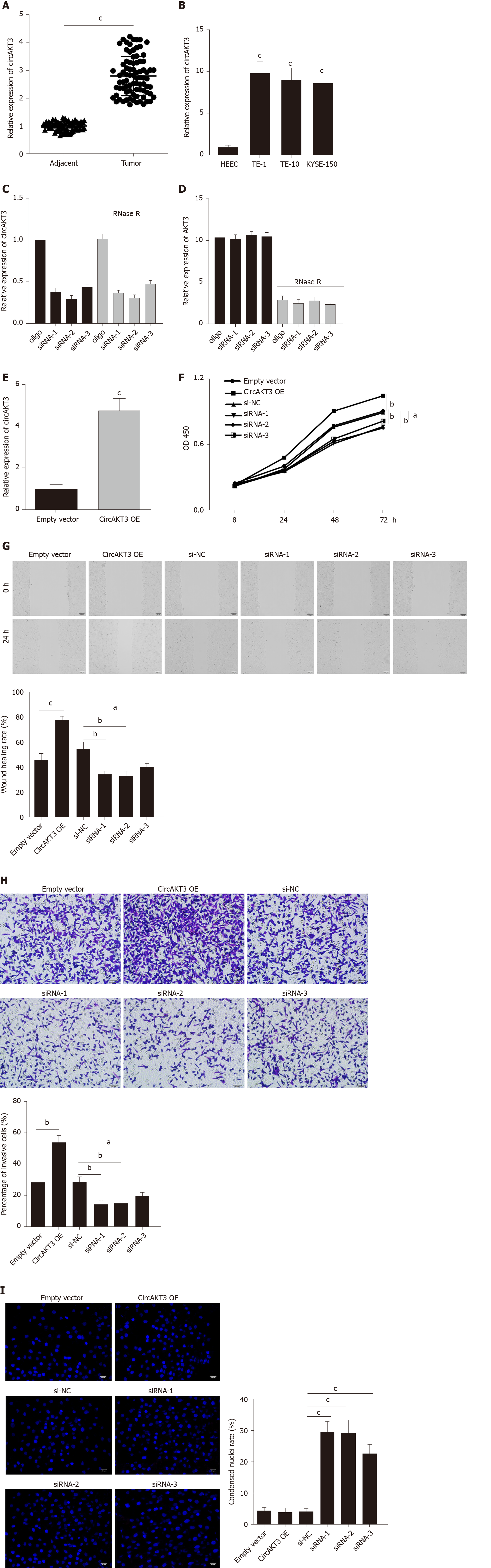
Figure 1 Circular RNA AKT3 promoted malignant phenotypes of TE-1 cells.
A: The expression of circular RNA AKT3 (circAKT3) in tissue samples; B: The expression of circAKT3 human normal esophageal epithelial cell line (HEEC) and human esophageal cancer cell lines; C and D: TE-1 cells were transfected with control oligo and circAKT3 siRNAs in the presence or absence of RNase R to detect the expression of circAKT3 and AKT3; E: The expression of circAKT3 in TE-1 cells with circAKT3 overexpression (OE); F: Cell viability of TE-1 cells as detected by the Cell Counting Kit-8 assay was performed to detect cell viability of TE-1 cells with indicated transfection; G: Migratory capacity of TE-1 cells with circAKT3 OE and knockdown; H: Invasive ability of TE-1 cells with circAKT3 OE and knockdown; I: Apoptosis of TE-1 cells with circAKT3 OE and knockdown by Hoechst staining. aP < 0.05; bP < 0.01; cP < 0.001. NC: Negative control; OD: Optical density.
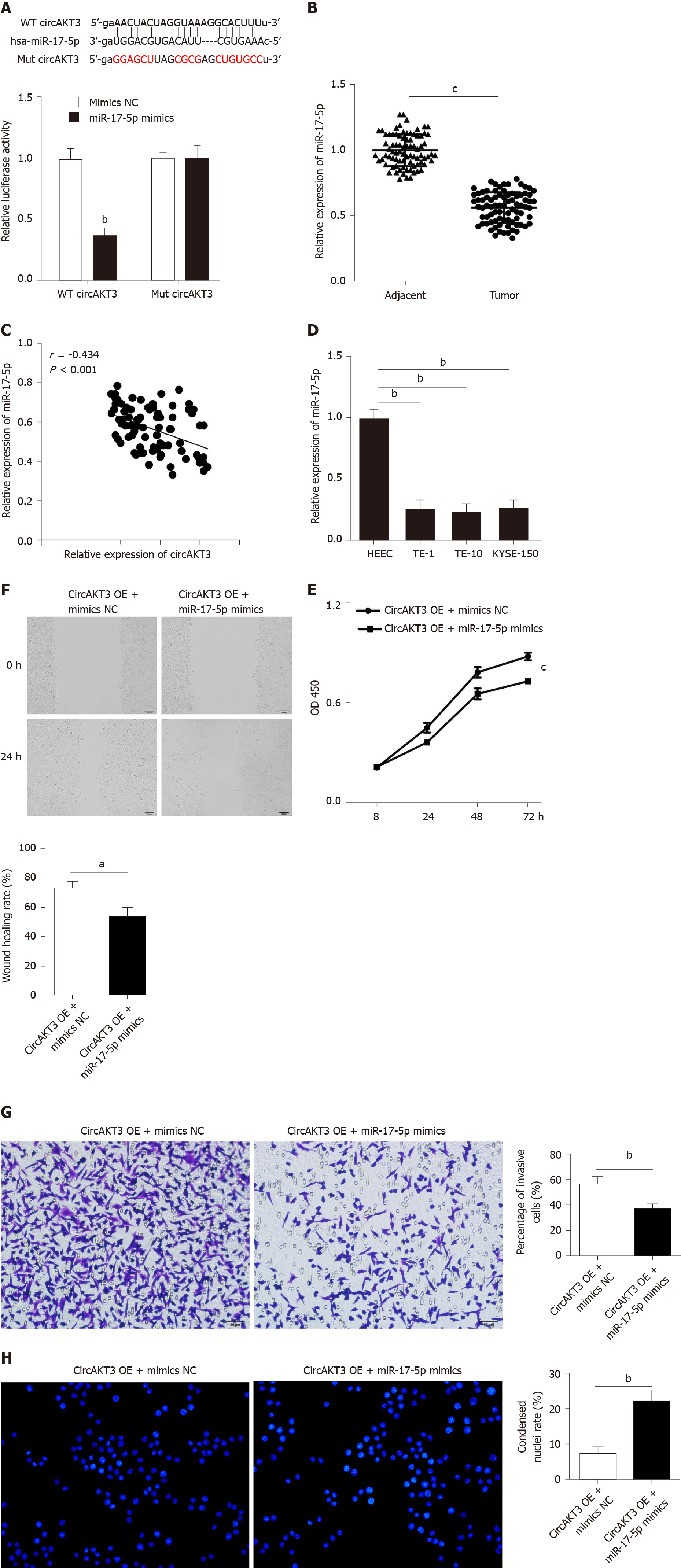
Figure 2 miR-17-5p acted as the downstream molecule of circular RNA AKT3 to antagonize the effects of circular RNA AKT3 on TE-1 cells.
A: Schematic of the miR-17-5p binding sites in wild-type (WT) circular RNA AKT3 (circAKT3) and the mutant (Mut) circAKT3 (up). Luciferase reporter assay was performed in HEK-293T cells to determine the binding ability between miR-17-5p and circAKT3 (down); B: The expression of miR-17-5p in tissue samples; C: CircAKT3 was negatively correlated with miR-17-5p in esophageal cancer tissue samples; D: miR-17-5p expression in indicated cell lines; E-H: Cell viability (E), migratory ability (F), invasive ability (G), and apoptosis (H) of circAKT3 overexpression (OE)-transfected TE-1 cells in the presence or absence of miR-17-5p mimics. aP < 0.05; bP < 0.01; cP < 0.001. HEEC: Human normal esophageal epithelial cell line; NC: Negative control; OD: Optical density.
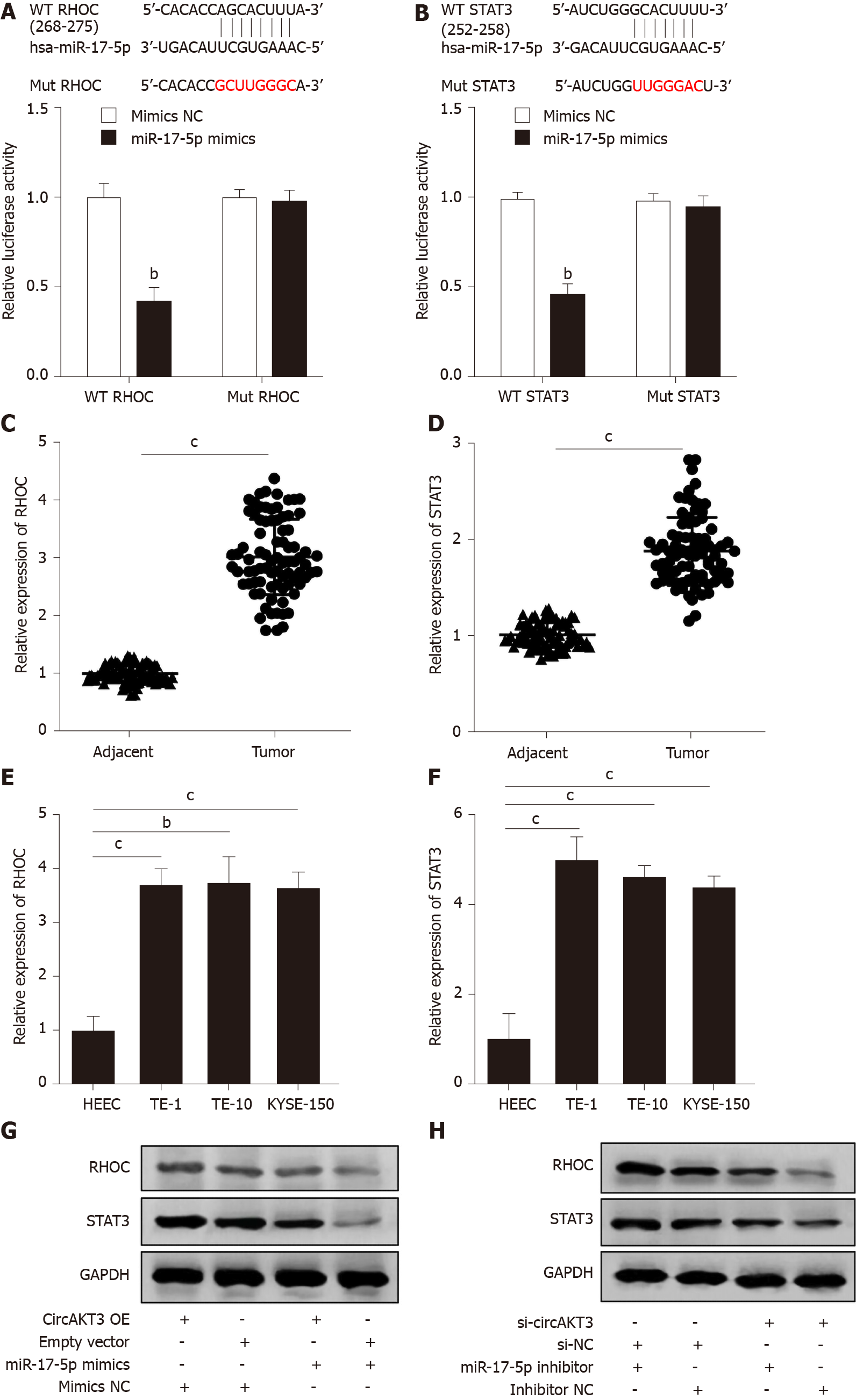
Figure 3 RHOC and STAT3 were modulated by the circular RNA AKT3/miR-17-5p axis.
A: Schematic of the miR-17-5p binding sites in wild-type (WT) RHOC and the mutant (Mut) RHOC (up). Luciferase reporter assay was performed in HEK-293T cells to determine the binding ability between miR-17-5p and RHOC (down); B: Schematic of the miR-17-5p binding site in WT STAT3 and the Mut STAT3 (up). Luciferase reporter assay was performed in HEK-293T cells to determine the binding ability between miR-17-5p and STAT3 (down); C and D: The expression of RHOC (C) and STAT3 (D) in tissue samples; E and F: The expression of RHOC (E) and STAT3 (F) in indicated cell lines; G and H: Protein level of RHOC and STAT3 in TE-1 cells with indicated transfection. bP < 0.01; cP < 0.001. circAKT3: Circular RNA AKT3; HEEC: Human normal esophageal epithelial cell line; NC: Negative control; OE: Overexpression.
Figure 4 Circular RNA AKT3 knockdown suppressed growth of esophageal cancer in vivo.
A: The expression of circular RNA AKT3 (circAKT3) in TE-1 cells with circAKT3 short hairpin (sh)RNA transfection; B and C: The size (B) and weight (C) of the xenograft tumor injected with circAKT3 shRNA-transfected TE-1 cells; D-G: The expression of circAKT3 (D), miR-17-5p (E), RHOC (F) and STAT3 (G) in the xenograft tumor injected with circAKT3 shRNA-transfected TE-1 cells by quantitative real time-polymerase chain reaction; H: The protein levels of RHOC and STAT3 in the xenograft tumor injected with circAKT3 shRNA-transfected TE-1 cells. cP < 0.001. sh-NC: Negative control shRNA; sh-circAKT3: Circular RNA AKT3 shRNA.
- Citation: Zang HL, Ji FJ, Ju HY, Tian XF. Circular RNA AKT3 governs malignant behaviors of esophageal cancer cells by sponging miR-17-5p. World J Gastroenterol 2021; 27(3): 240-254
- URL: https://www.wjgnet.com/1007-9327/full/v27/i3/240.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i3.240