Copyright
©The Author(s) 2021.
World J Gastroenterol. Jun 14, 2021; 27(22): 3022-3036
Published online Jun 14, 2021. doi: 10.3748/wjg.v27.i22.3022
Published online Jun 14, 2021. doi: 10.3748/wjg.v27.i22.3022
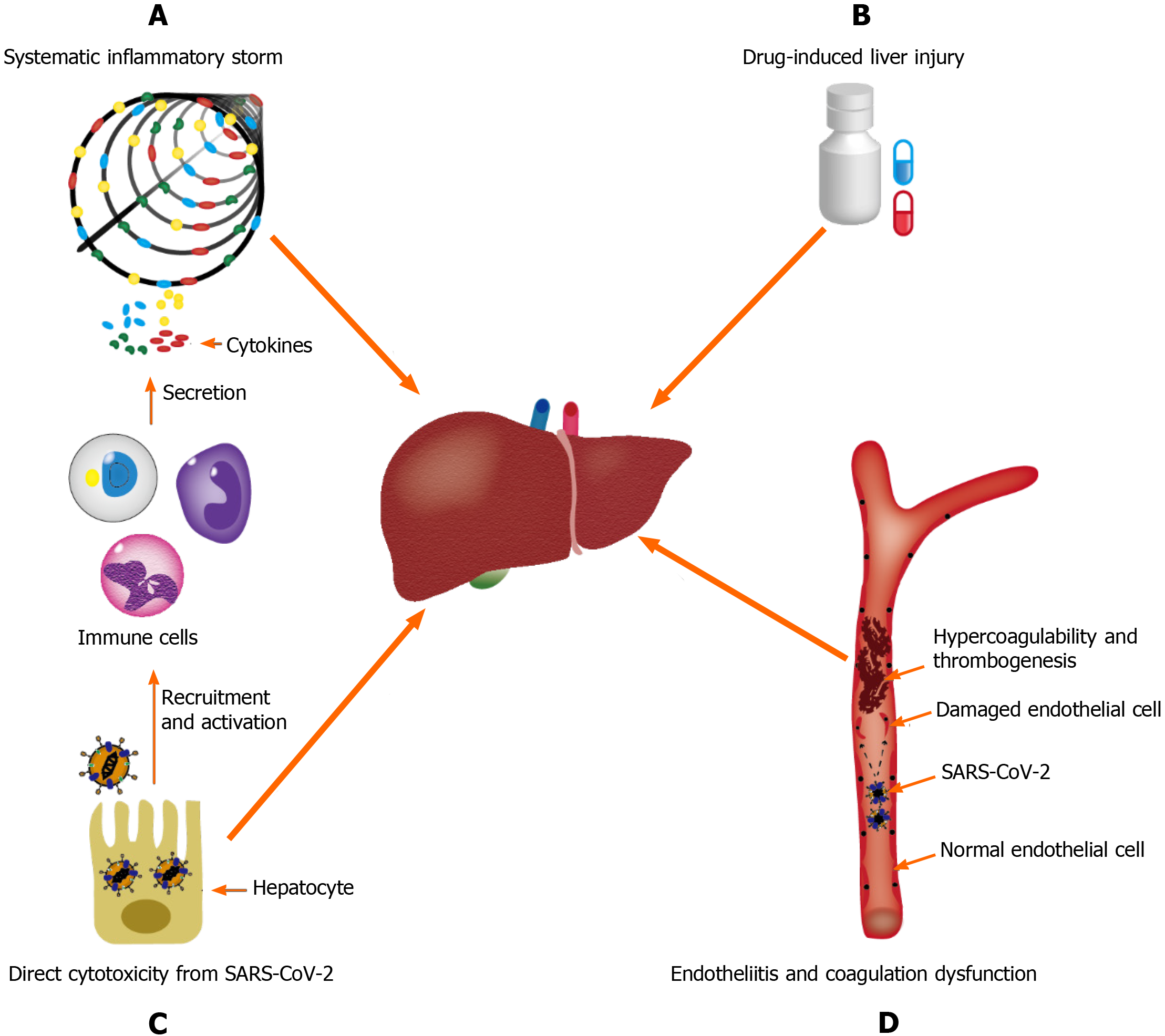
Figure 1 Underlying mechanisms of coronavirus disease-19-assocaited liver injury.
A: Systematic inflammatory storm, which is generated by abnormal activation of the immune system; B: Drug-induced liver injury; C: Endotheliitis and coagulation dysfunction; D: Direct cytotoxicity from severe acute respiratory syndrome coronavirus 2. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
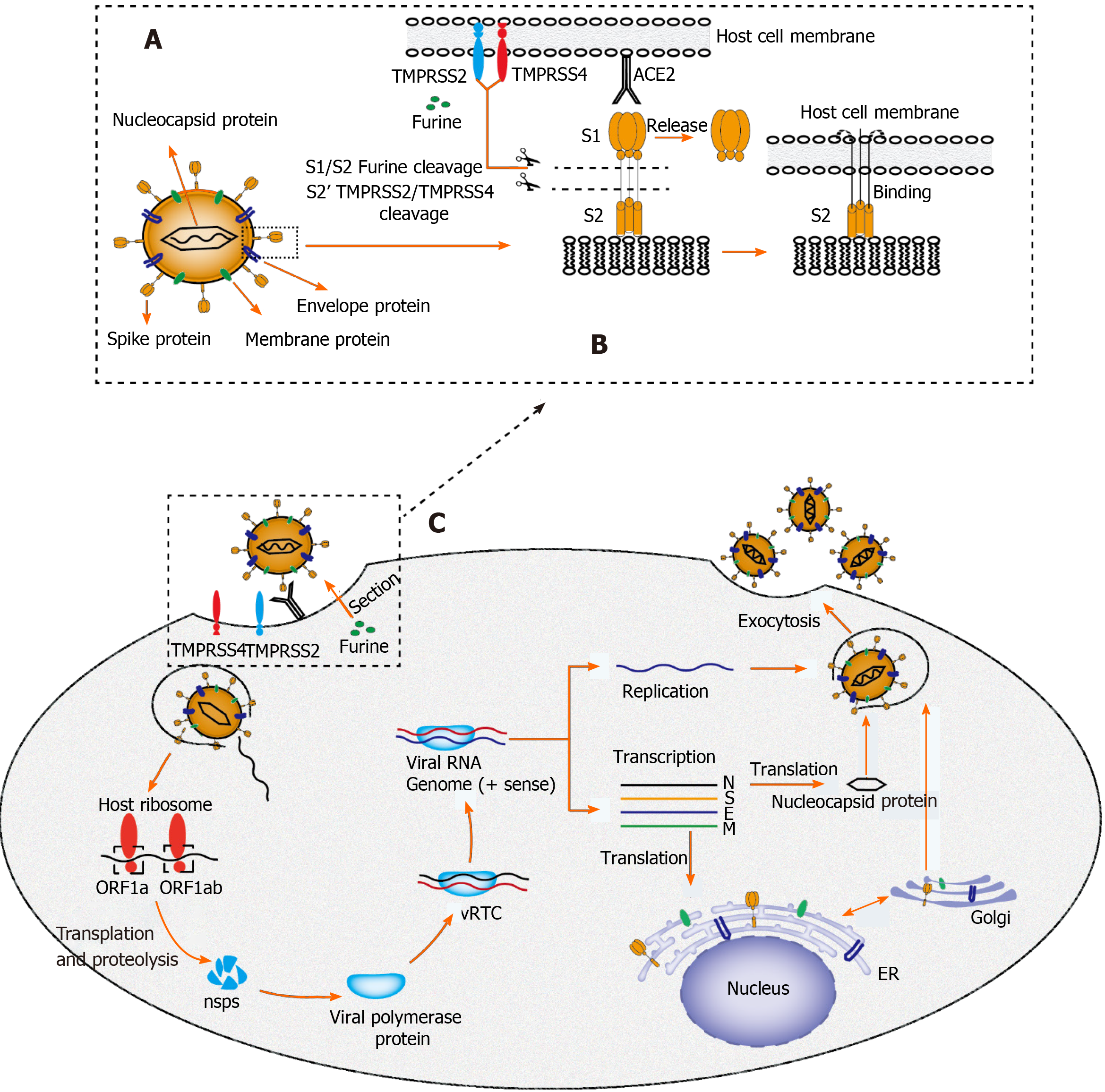
Figure 2 Proposed structure diagram of severe acute respiratory syndrome coronavirus 2 and its life cycle in host cells.
A: Structural sketch of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); B: Recognition and entry of SARS-CoV-2 into host cell. Transmembrane spike (S) glycoprotein on the surface of SARS-CoV-2 forms a homotrimer to recognize the human host angiotensin converting enzyme 2 (ACE2) receptor. The S protein is specifically cleaved by two mucose-specific serine proteases [recombinant transmembrane protease serine 2 (TMPRSS2) and TMPRSS4] and furine. The subunit of S protein (S1) is released, and another subunit (S2) is exposed and mediates the viral entry into host cells; C: Life cycle of SARS-CoV-2 in host cells. First, the S protein of SARS-CoV-2 binds to ACE2 to form an S protein-ACE2 complex, which directly mediates the cellular entry of virus and the process is facilitated by TMPRSS2, TMPRSS4, and furine. Second, viral RNA is released into host cytoplasm. Open reading frame (ORF) 1a and ORF1ab are translated into large polyproteins by host ribosome, which are further proteolytically cleaved into 16 non-structural proteins (nsps). Viral polymerase protein is assembled by nsps and viral replication/transcription complex (vRTC) is subsequnently formed by polymerase protein and genomic RNA. Third, a negative sense viral RNA is synthesized and used as a template to replicate progeny (+) sense viral genome and transcribes to form various mRNAs. The nucleocapsid protein is translated in the cytoplasm, whereas the S protein, membrane (M) protein, and envelope (E) protein are translated in the endoplasmic reticulum and transported to the Golgi apparatus for further packaging. Finally, a completely new viral particle is assembled by viral RNA-nucleocapsid complex and S, M, and E proteins in endoplasmic reticulum–Golgi intermediate compartment and is released from host cell via exocytosis. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; M: Membrane; E: Envelope; S: Spike; ER: Endoplasmic reticulum; vRTC: Viral replication/transcription complex; ORF: Open reading frame; TMPRSS: Transmembrane protease serine; ACE2: Angiotensin converting enzyme 2.
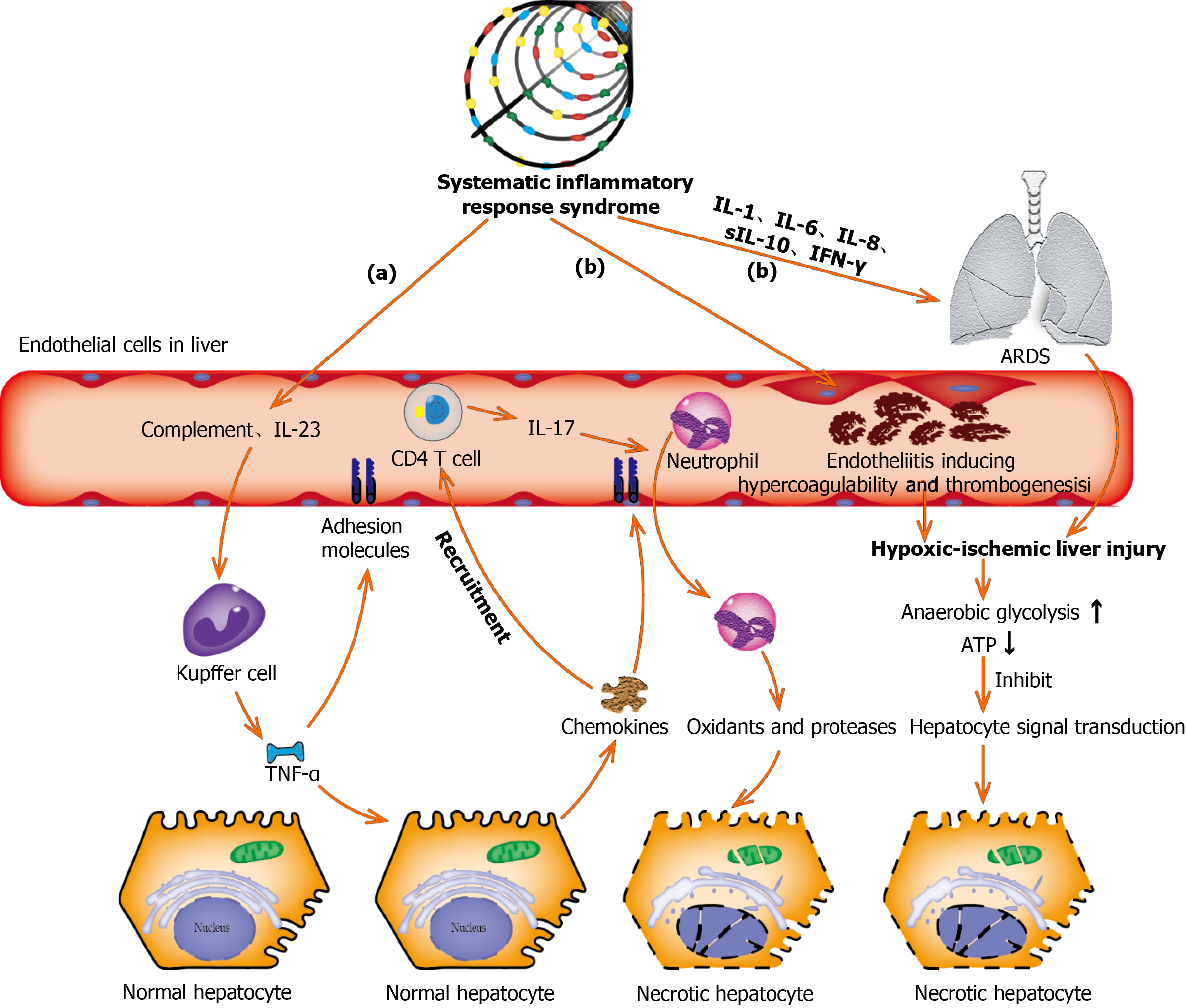
Figure 3 Underlying molecular mechanisms of coronavirus disease-19-associated liver injury caused by systematic inflammatory response syndrome and hypoxic ischemia.
(a): Complement and interleukin-23 are released into the blood during the systemic inflammation, which subsequently activate Kupffer cells and induce their production of tumor necrosis factor α (TNF-α). As a pro-inflammatory cytokine, TNF-α aggravates the inflammation responses by up-regulating the expression of endothelial cell adhesion molecules and inducing hepatocytes to secrete chemokines. Under the induction of chemokines, CD4 T cells and neutrophils are rapidly recruited to the liver, in which CD4 T cells assist mucosal molecules to promote neutrophils into the liver parenchyma. Finally, neutrophils directly damage liver cells by releasing oxidants and proteases, leading to necrotic cell death; (b): Acute respiratory distress syndrome and endotheliitis are the two main causes leading to hypoxic-ischemic liver injury in the period of systematic inflammatory response syndrome. Increased anaerobic glycolysis leads to a decrease in ATP production, which ultimately leads to the death of hepatocytes by inhibiting hepatocyte signal transduction. ARDS: Acute respiratory distress syndrome; TNF-α: Tumor necrosis factor α; IL: Interleukin; IFN-γ: Interferon-γ.
- Citation: Cai Y, Ye LP, Song YQ, Mao XL, Wang L, Jiang YZ, Que WT, Li SW. Liver injury in COVID-19: Detection, pathogenesis, and treatment. World J Gastroenterol 2021; 27(22): 3022-3036
- URL: https://www.wjgnet.com/1007-9327/full/v27/i22/3022.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i22.3022