Copyright
©The Author(s) 2021.
World J Gastroenterol. Jun 14, 2021; 27(22): 3022-3036
Published online Jun 14, 2021. doi: 10.3748/wjg.v27.i22.3022
Published online Jun 14, 2021. doi: 10.3748/wjg.v27.i22.3022
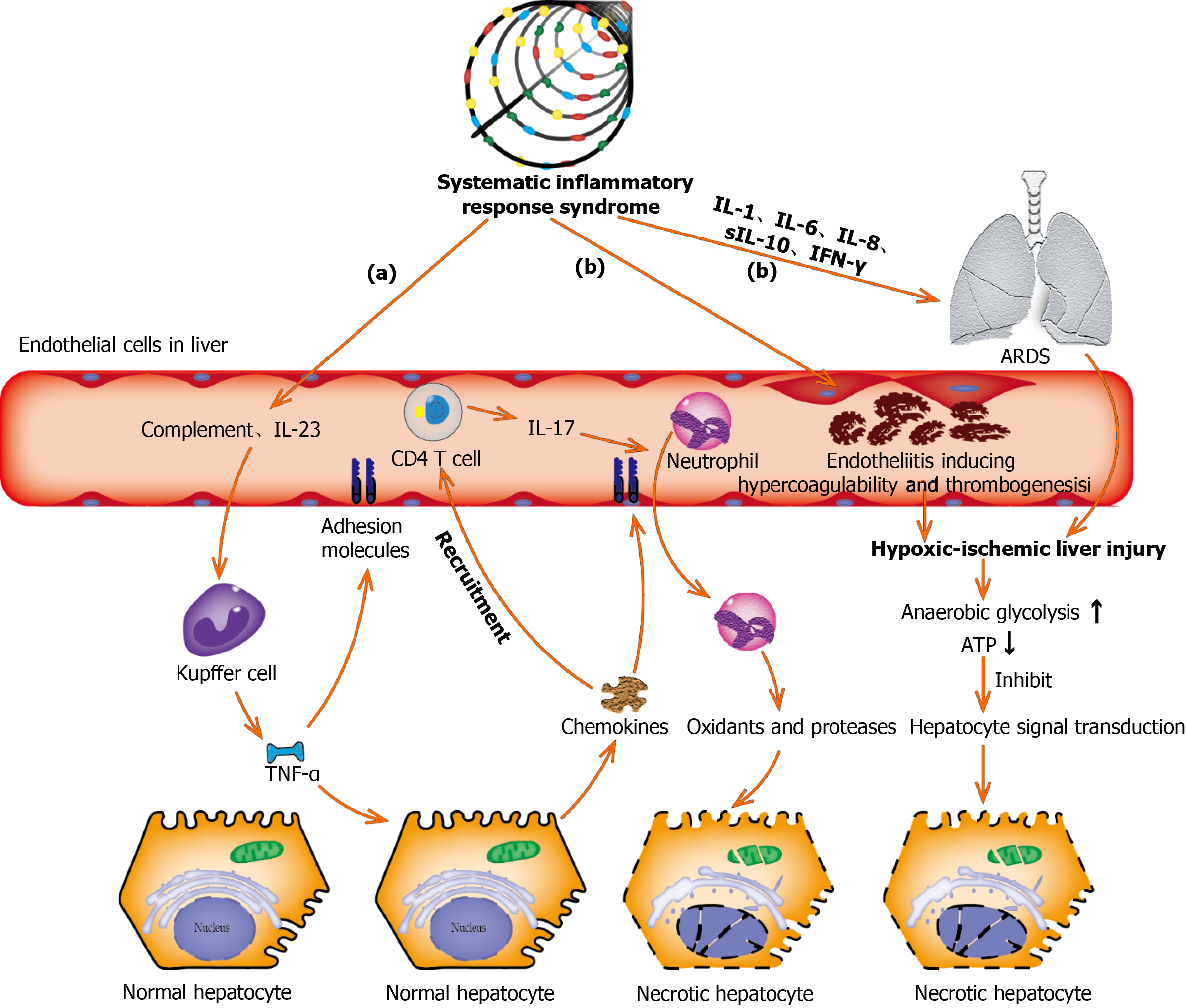
Figure 3 Underlying molecular mechanisms of coronavirus disease-19-associated liver injury caused by systematic inflammatory response syndrome and hypoxic ischemia.
(a): Complement and interleukin-23 are released into the blood during the systemic inflammation, which subsequently activate Kupffer cells and induce their production of tumor necrosis factor α (TNF-α). As a pro-inflammatory cytokine, TNF-α aggravates the inflammation responses by up-regulating the expression of endothelial cell adhesion molecules and inducing hepatocytes to secrete chemokines. Under the induction of chemokines, CD4 T cells and neutrophils are rapidly recruited to the liver, in which CD4 T cells assist mucosal molecules to promote neutrophils into the liver parenchyma. Finally, neutrophils directly damage liver cells by releasing oxidants and proteases, leading to necrotic cell death; (b): Acute respiratory distress syndrome and endotheliitis are the two main causes leading to hypoxic-ischemic liver injury in the period of systematic inflammatory response syndrome. Increased anaerobic glycolysis leads to a decrease in ATP production, which ultimately leads to the death of hepatocytes by inhibiting hepatocyte signal transduction. ARDS: Acute respiratory distress syndrome; TNF-α: Tumor necrosis factor α; IL: Interleukin; IFN-γ: Interferon-γ.
- Citation: Cai Y, Ye LP, Song YQ, Mao XL, Wang L, Jiang YZ, Que WT, Li SW. Liver injury in COVID-19: Detection, pathogenesis, and treatment. World J Gastroenterol 2021; 27(22): 3022-3036
- URL: https://www.wjgnet.com/1007-9327/full/v27/i22/3022.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i22.3022