Copyright
©The Author(s) 2020.
World J Gastroenterol. Feb 14, 2020; 26(6): 562-597
Published online Feb 14, 2020. doi: 10.3748/wjg.v26.i6.562
Published online Feb 14, 2020. doi: 10.3748/wjg.v26.i6.562
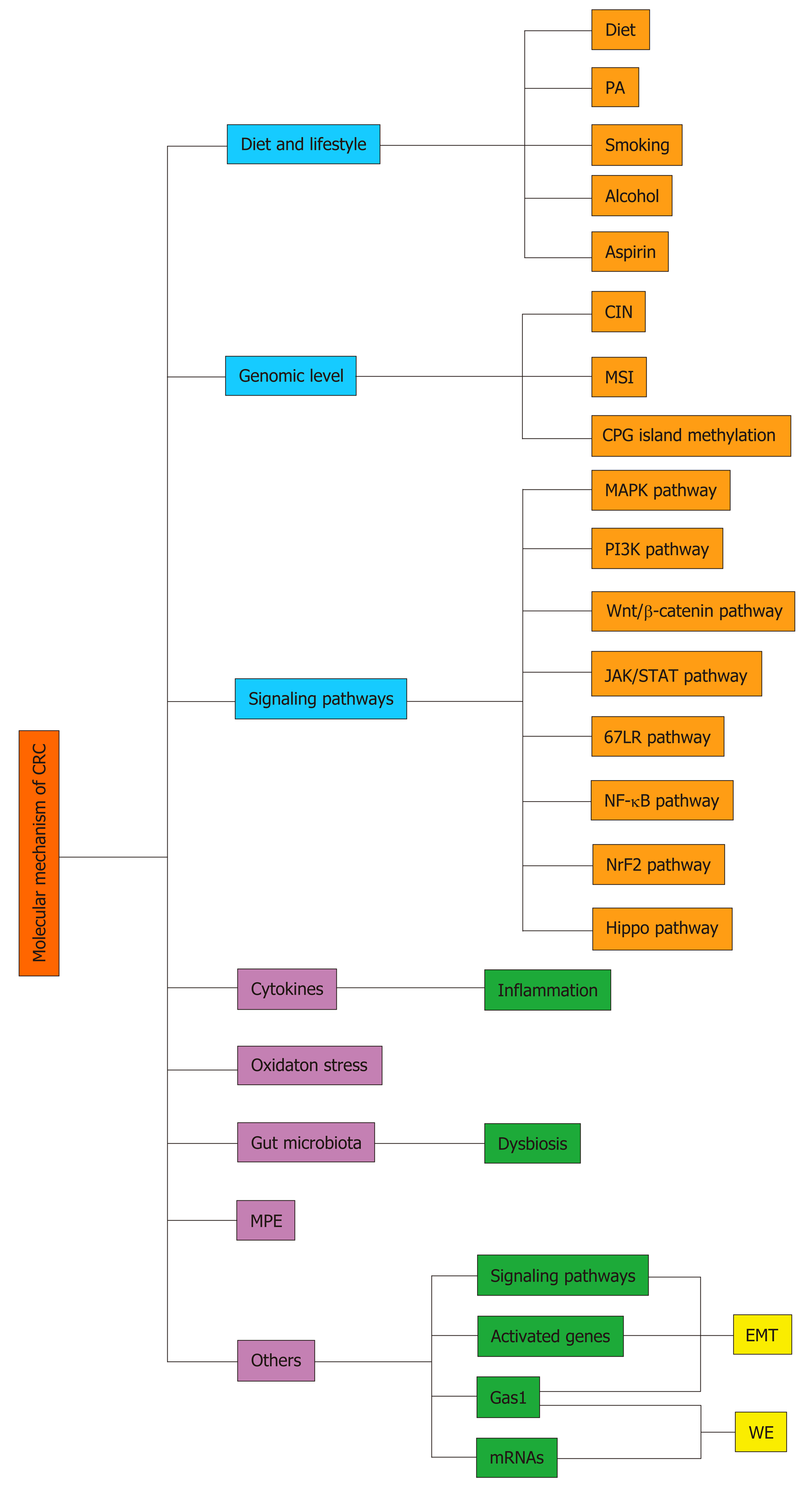
Figure 1 The general molecular mechanisms of colorectal cancer.
CRC: Colorectal cancer; MPE: Molecular pathological epidemiology; PA: Physical activity; CIN: Chromosomal instability; 67LR: 67 kDa laminin receptor; MSI: Microsatellite instability; EMT: Epithelial-to-mesenchymal transition; WE: Warburg effect.
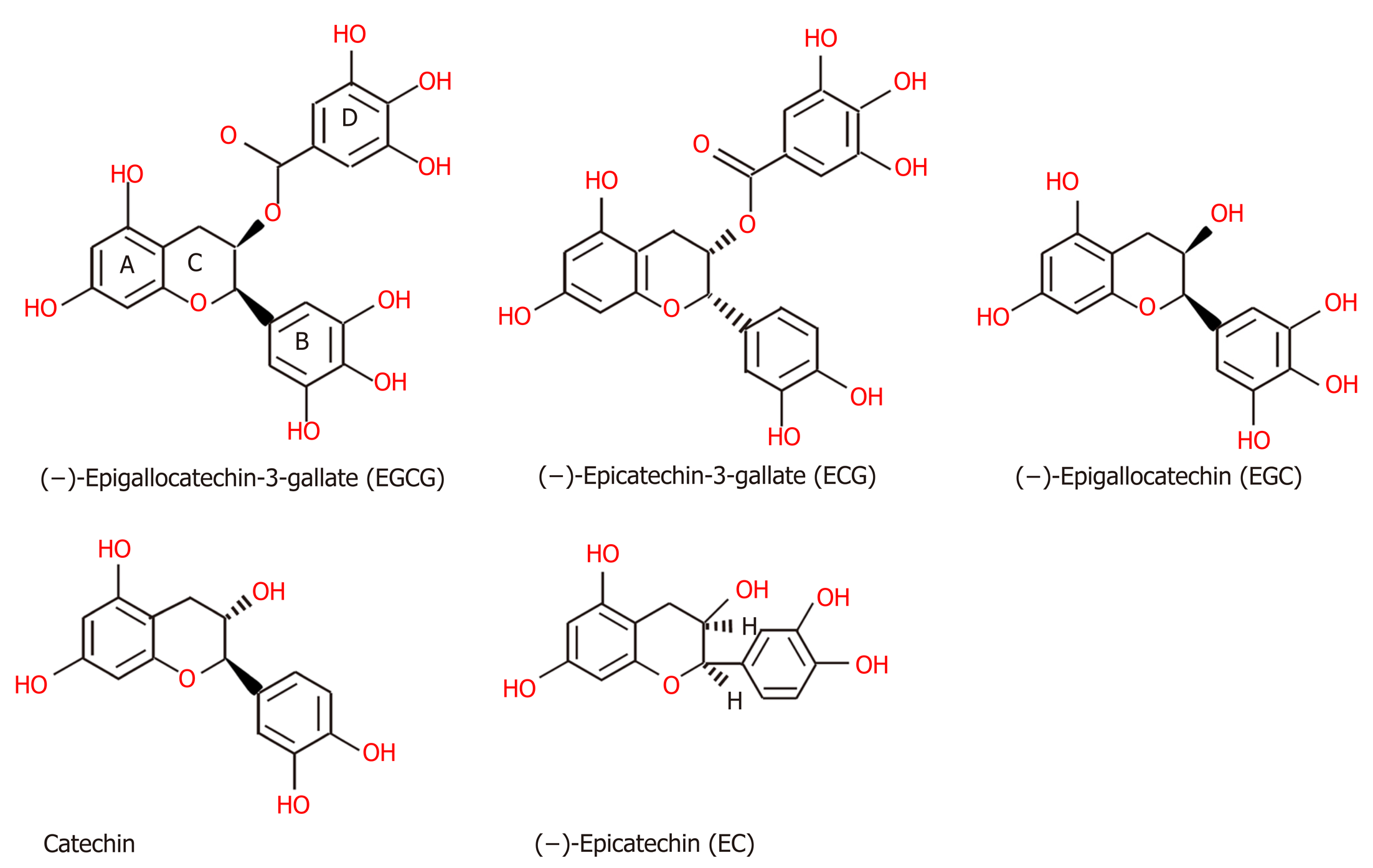
Figure 2 The structures of green tea polyphenols, including (−)-epigallocatechin-3-gallate, (−)-epicatechin-3-gallate, (−)-epigallocatechin, catechin, (−)-epicatechin[146].
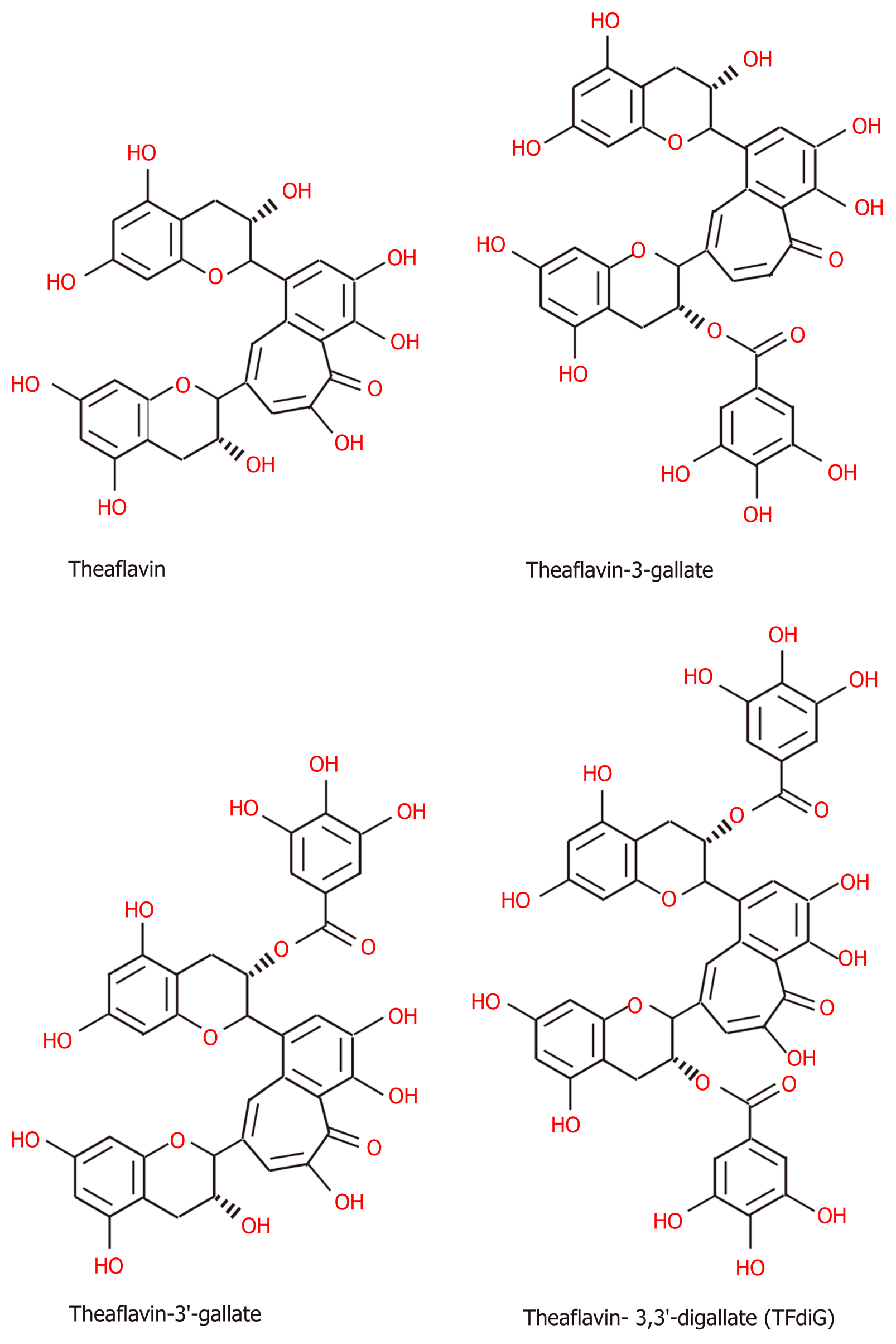
Figure 3 The structures of black tea polyphenols, including, theaflavin, theaflavin-3-gallate, theaflavin-3'-gallate and theaflavin- 3,3'-digallate[150].
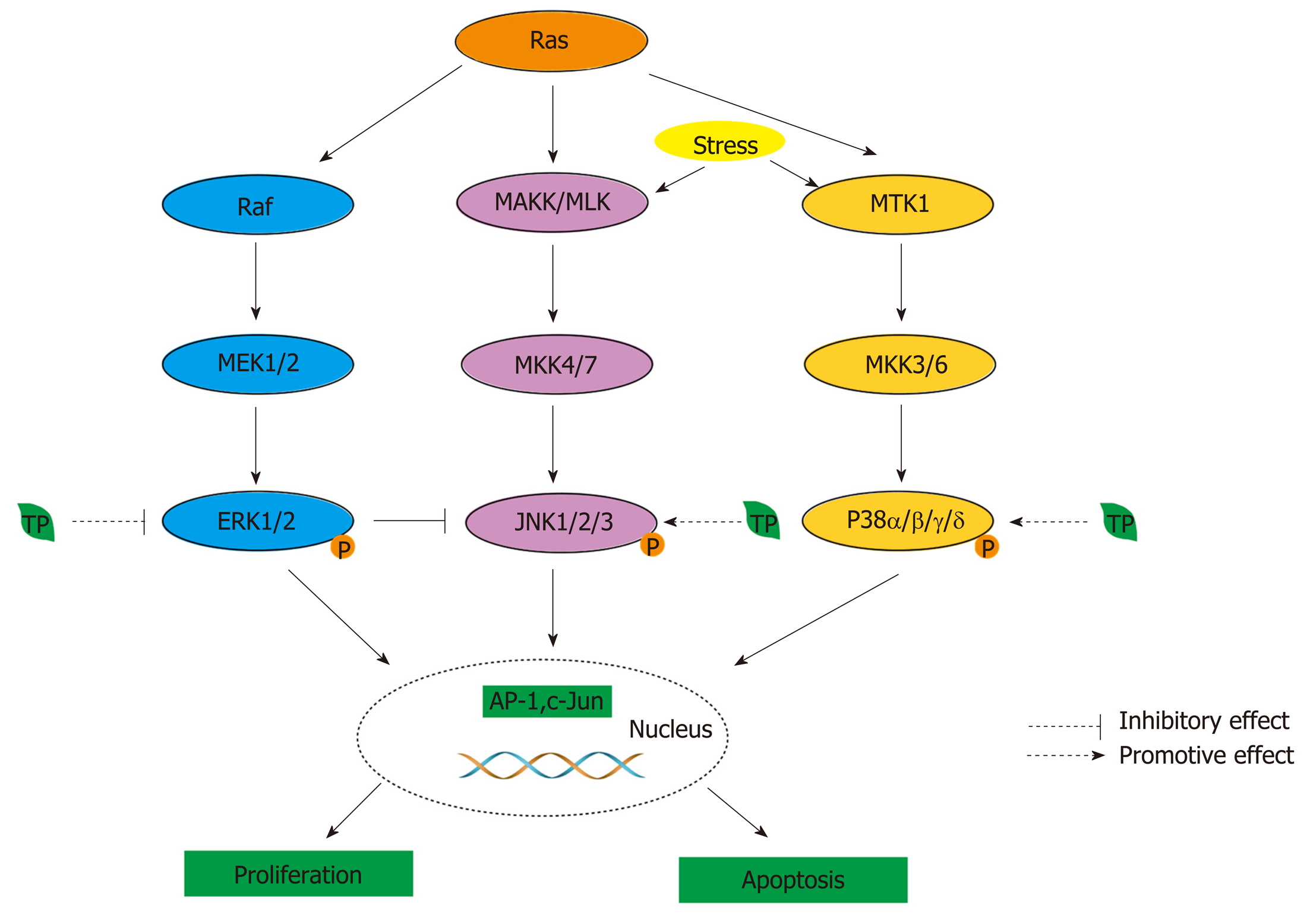
Figure 4 MAPK activity is regulated through three-tiered cascades composed of a MAPK, MAPK kinase (MAPKK, MKK, or MEK), and an MAPKK kinase or MEK kinase (MAPKKK or MEKK)[190].
Four groups of MAPKs are extracellular signal-related kinase (ERK)-1/2, Jun amino-terminal kinase (JNK1/2/3), p38 proteins (p38α/β/γ/δ), and ERK5, which are activated by specific MAPKKs: MEK1/2, MKK4/7 (JNKK1/2), MKK3/6, and MEK5, respectively[190]. MAPKKKs, the upstream targets of MAPKKs, contain Raf in the ERK pathway, MEK kinase (MEKK) and mixed-lineage kinase (MLK) in the JNK pathway, and MTK1 (or MEKK4) and apoptosis signal-regulating kinase 1 in the p38 pathway. Moreover, phosphorylated MAPKs can activate transcription factors, such as AP-1. Tea polyphenols (TPs) can modulate MAPK pathways. In the ERK pathway, TPs can inhibit the activity of transcription factors, such as AP-1, by inhibiting the phosphorylation of ERK1/2. In the JNK and p38 pathway, TPs can promote the activation of JNK1/2 and p38 to induce cell death. In addition, the blockade of ERK can also lead to the activation of the JNK pathway.
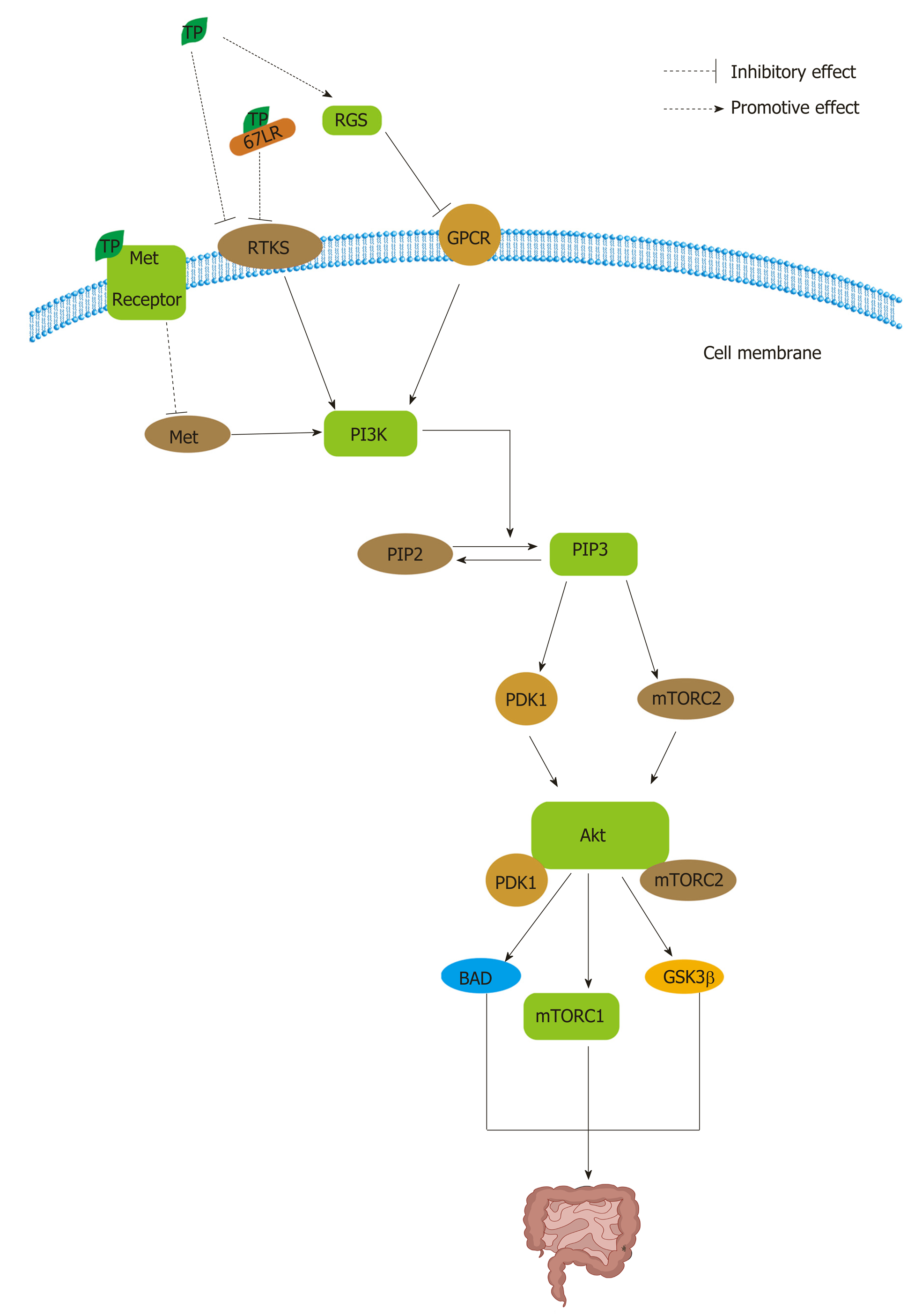
Figure 5 The class I PI3Ks activated by receptor tyrosine kinases, such as epidermal growth factor receptor, can phosphorylate PI 4,5-bisphosphate to yield PI 3,4,5-triphosphate in the cell membrane[203].
The activation of PI3K results in the phosphorylation of two key residues on Akt1, T308 in the activation loop (or T-loop) of the catalytic protein kinase core and S473 in a C-terminal hydrophobic motif[201]. PDK1 at T308 and mTOR complex 2 (mTORC2) at S473 can activate Akt[203]. Phosphorylated Akt activates a multitude of downstream targets, such as the mTOR complex 1 (mTORC1), BAD, GSK3β. Tea polyphenols (TPs) can inhibit PI3K/Akt signaling by interrupting receptor tyrosine kinases (RTKs) and G protein-coupled receptors. TPs can inhibit RTKs not only directly but also indirectly by binding to 67 kDa laminin receptor. Moreover, TPs can activate the regulators of G protein signaling to negatively regulate the pathway. In addition, TPs can bind to the Met receptor to inhibit PI3K activation. PIP2: PI 4,5-bisphosphate; PIP3: PI 3,4,5-triphosphate; TP: Tea polyphenol; RGS: Regulators of G protein signaling; 67LR: 67 kDa laminin receptor; GPCR: G protein-coupled receptor.
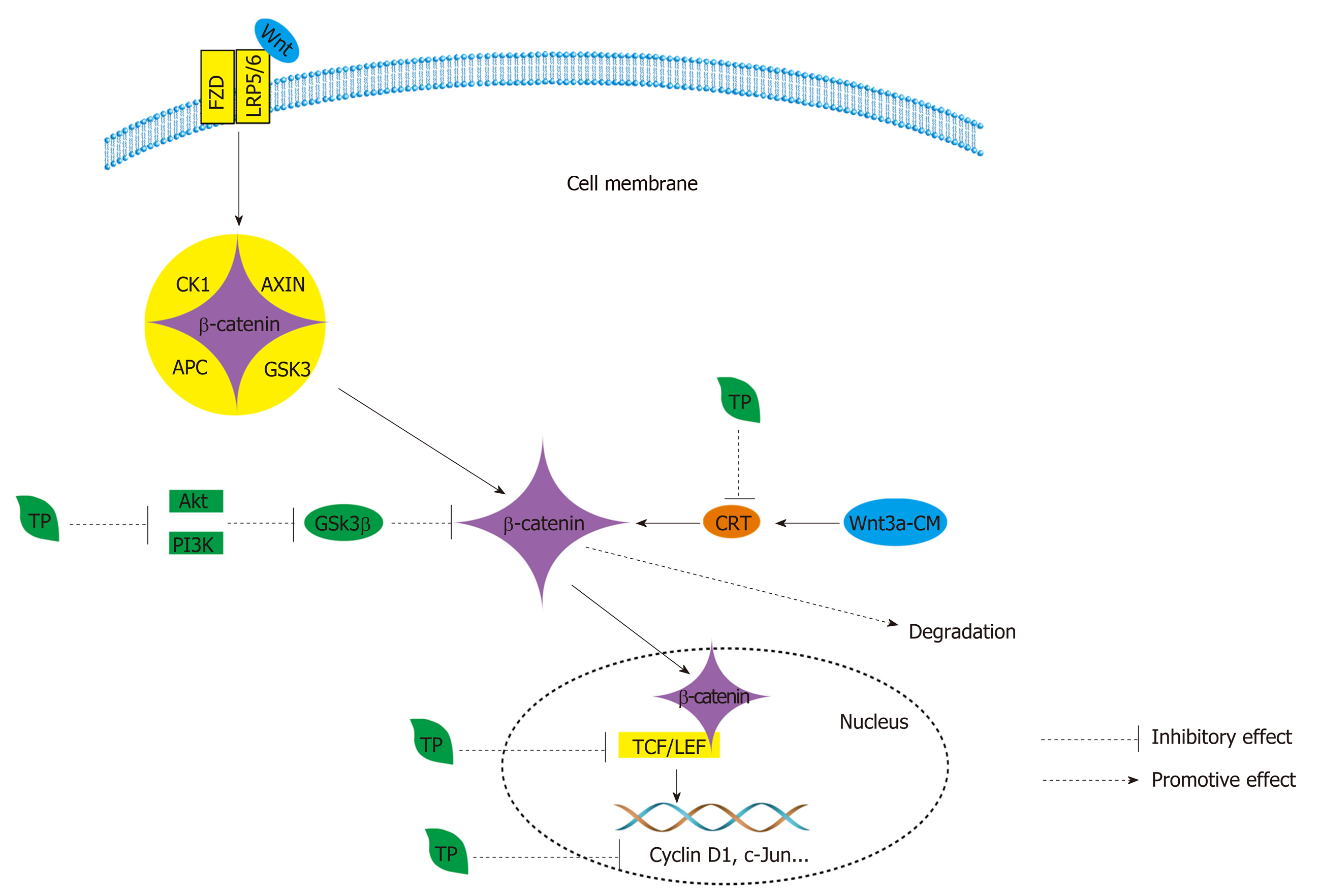
Figure 6 Wnt signaling is regulated by the cytoplasmic β-catenin destruction complex, which consists of the core proteins AXIN, adenomatous polyposis coli, casein kinase 1, and glycogen synthase kinase 3[209].
Wnt ligands bind to the frizzled and low-density-lipoprotein-related protein 5/6 coreceptor complex and cause the accumulation and nuclear translocation of β-catenin. In the nucleus, β-catenin engages the T cell factor (TCF)/lymphoid enhancer-binding factor transcription factors to activate the Wnt transcriptional program[210]. In the Wnt/β-catenin pathway, Tea polyphenols (TPs) can inhibit β-catenin/TCF-4 activity and reduce β-catenin protein expression and further reduce the expression of two downstream signaling targets, namely, cyclin D1 and c-Jun. Black TPs can also suppress the accumulation of β-catenin by suppressing the phosphorylation of GSK3β by decreasing the activation of PI3Knase and Akt. Furthermore, TPs also promote the degradation of β-catenin by inhibiting β-catenin response transcription. APC: Adenomatous polyposis coli; CK1: Casein kinase 1; GSK3: Glycogen synthase kinase 3; TCF: T cell factor; TP: Tea polyphenol; LEF: Lymphoid enhancer-binding factor; LRP5/6: Low-density lipoprotein-related protein 5/6; CRT: β-catenin response transcription.
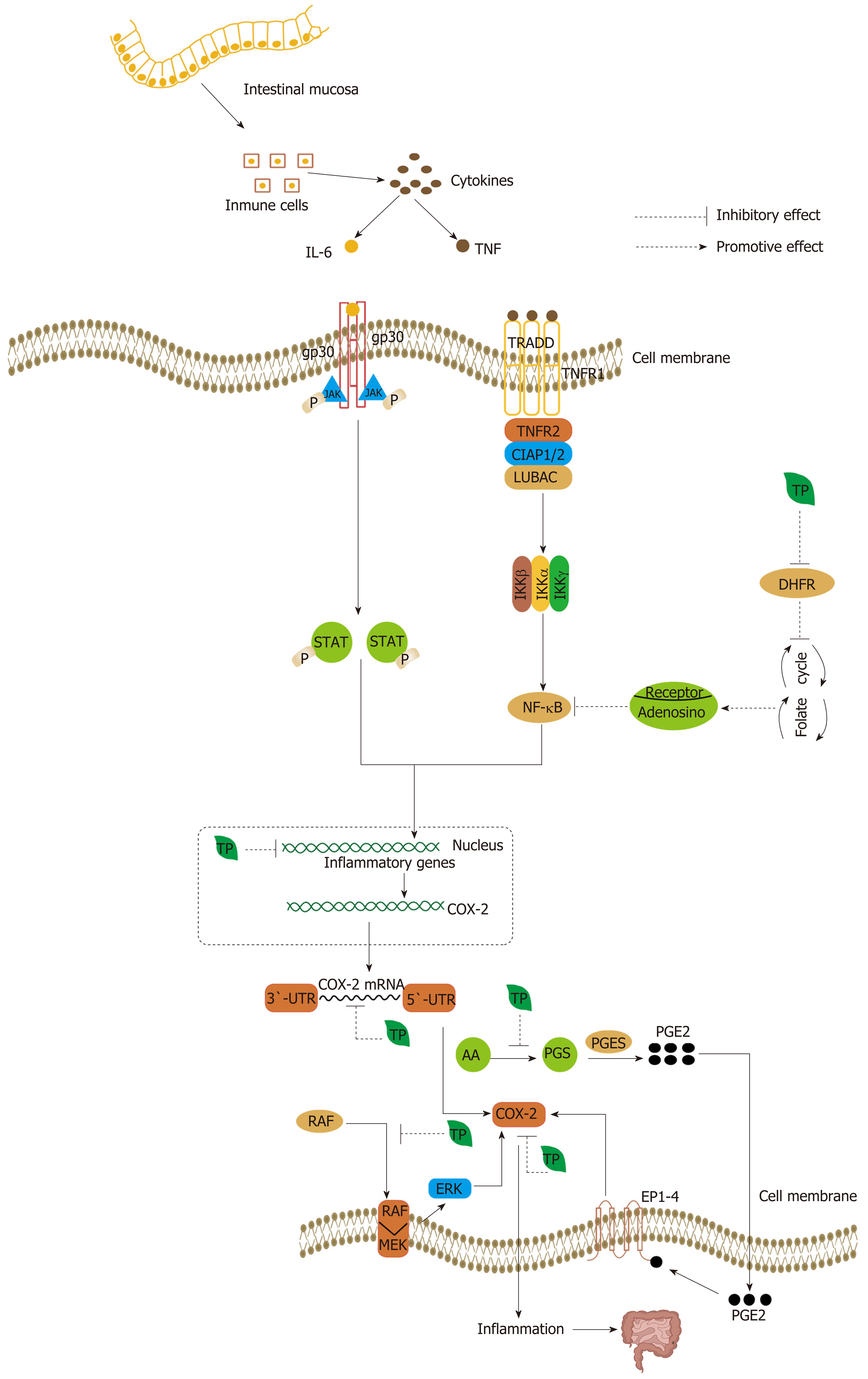
Figure 7 Tumor necrosis factor from immune cells can activate nuclear factor-kappa B, a central activator that induces the expression of inflammatory genes, such as COX-2, tumor necrosis factor-alpha, inducible nitric oxide synthase, and ICAM-1, and tumor cell survival, proliferation, invasion, angiogenesis, and metastasis are affected when tumor necrosis factor binds to tumor necrosis factor receptor 1 and assembles with tumor necrosis factor receptor 1-associated death domain protein, tumor necrosis factor receptor-associated factor 2, cellular inhibitor of apoptosis protein 1 or 2, and linear ubiquitin chain assembly complex[219,230].
Similarly, another cytokine, interleukin (IL)-6, is also secreted in the tumor microenvironment. The binding of IL-6 to the IL-6 receptor activates STAT3, a major oncogenic transcription factor[229]. After binding to the IL-6 receptor at the cell surface, gp130 dimerization and activation and the transphosphorylation of JAKs are achieved and then contribute to the phosphorylation of STAT3, causing its translocation from the cytoplasm to the nucleus, where it modulates the expression of a variety of genes[229]. Tea polyphenols (TPs) can inhibit inflammatory gene expression caused by cytokines secreted by immune cells in colorectal cancer, with the suppression of COX-2 being particularly important. TPs can inhibit COX-2 in a multilevel process, majorly at the transcriptional and post-transcriptional level. In addition, (-)-epigallocatechin-3-gallate (EGCG) can suppress the expression of nuclear factor-kappa B (NF-κB) through interruption of folate cycle. EGCG can inhibit dihydrofolate reductase and thereby interfere with the folate cycle, causing the release of adenosine. Adenosine binding to specific receptors inhibits the activation of NF-κB. In addition, prostaglandin E2 (PGE2) can also activate COX-2 by stimulating EP receptor (EP1-4) signaling, with the subsequent induction of colorectal cancer onset. Moreover, TPs can inhibit the synthesis of PGE2 and thereby suppress the expression of COX-2. TNF: Tumor necrosis factor; TRADD: TNFR1-associated death domain protein; TRAF2: TNFR-associated factor 2; LUBAC: Linear ubiquitin chain assembly complex; IL-6: Interleukin 6; TP: Tea polyphenol.
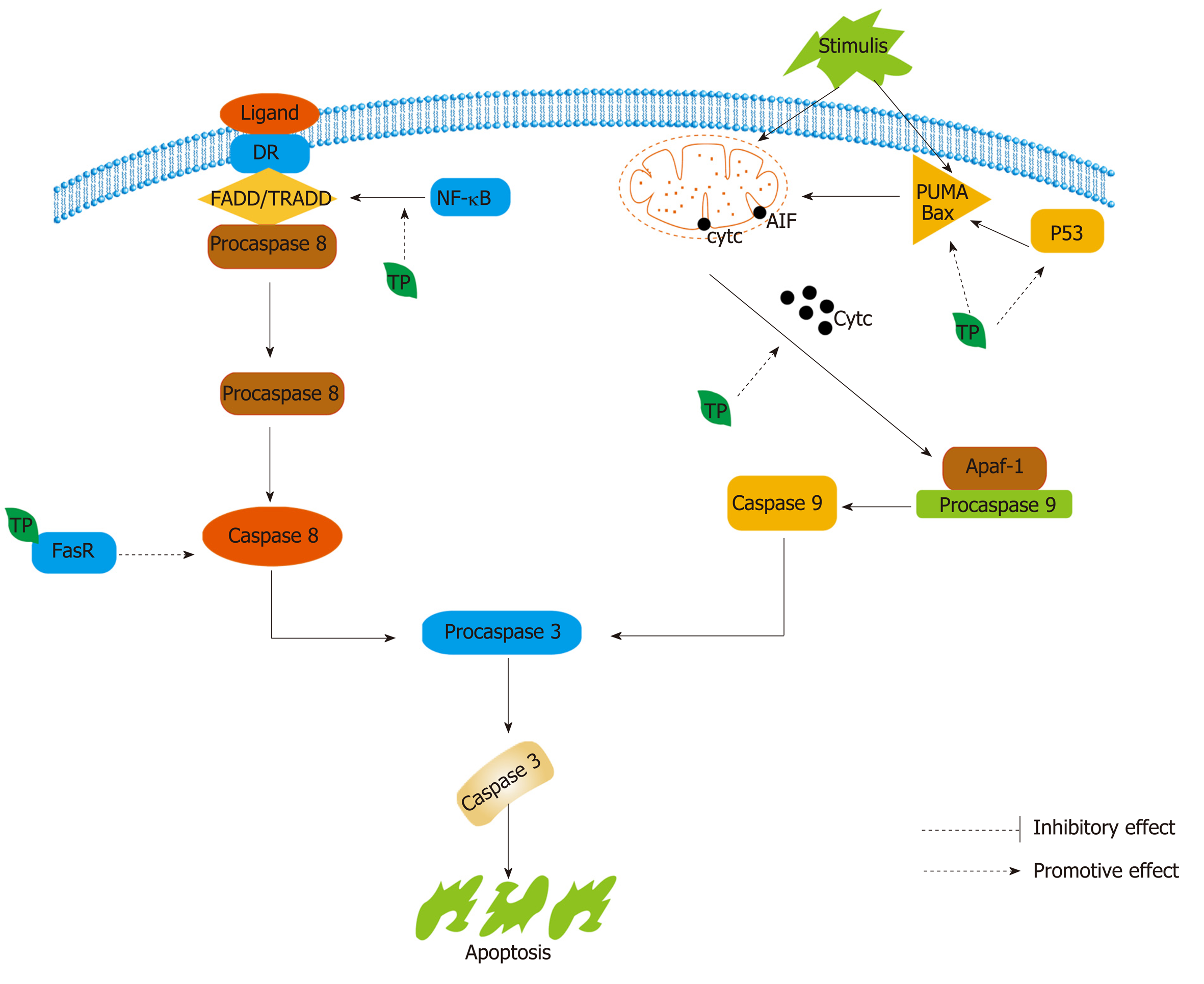
Figure 8 Both extrinsic and intrinsic pathways converge in the same terminal execution pathway, which is initiated by the cleavage of caspase-3.
In the extrinsic pathway, FasL/FasR and tumor necrosis factor-alpha (TNF-α)/ tumor necrosis factor receptor (TNFR)1 associate with the adapter protein called Fas-associated death domain (FADD), and procaspase-8 and TNF-α/TNFR1 also need to bind to TNFR-associated death domain to initiate the execution pathway[253]. In the intrinsic pathway, all stimuli can change the inner mitochondrial membrane to initiate the mitochondrial permeability transition and release two main groups of normally sequestered pro-apoptotic proteins, such as cytochrome c, from the mitochondria into the cytosol[255]. These proteins activate procaspase-9 and caspase-9 to activate the caspase-dependent mitochondrial pathway[253]. Tea polyphenols (TPs) can promote apoptosis of tumor cells. In the intrinsic pathway, TPs can up-regulate p53, Bax, and PUMA, which are proteins that promote cell death. In addition, TPs can also induce the release of cytochrome c from the mitochondria into the cytosol. In the extrinsic pathway, TPs induce apoptosis, mediated by caspase-8, through a FADD-dependent pathway. FADD: Fas-associated death domain; TRADD: TNFR-associated death domain; TP: Tea polyphenol.
- Citation: Wang ST, Cui WQ, Pan D, Jiang M, Chang B, Sang LX. Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer. World J Gastroenterol 2020; 26(6): 562-597
- URL: https://www.wjgnet.com/1007-9327/full/v26/i6/562.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i6.562