Copyright
©The Author(s) 2019.
World J Gastroenterol. Jul 14, 2019; 25(26): 3299-3312
Published online Jul 14, 2019. doi: 10.3748/wjg.v25.i26.3299
Published online Jul 14, 2019. doi: 10.3748/wjg.v25.i26.3299
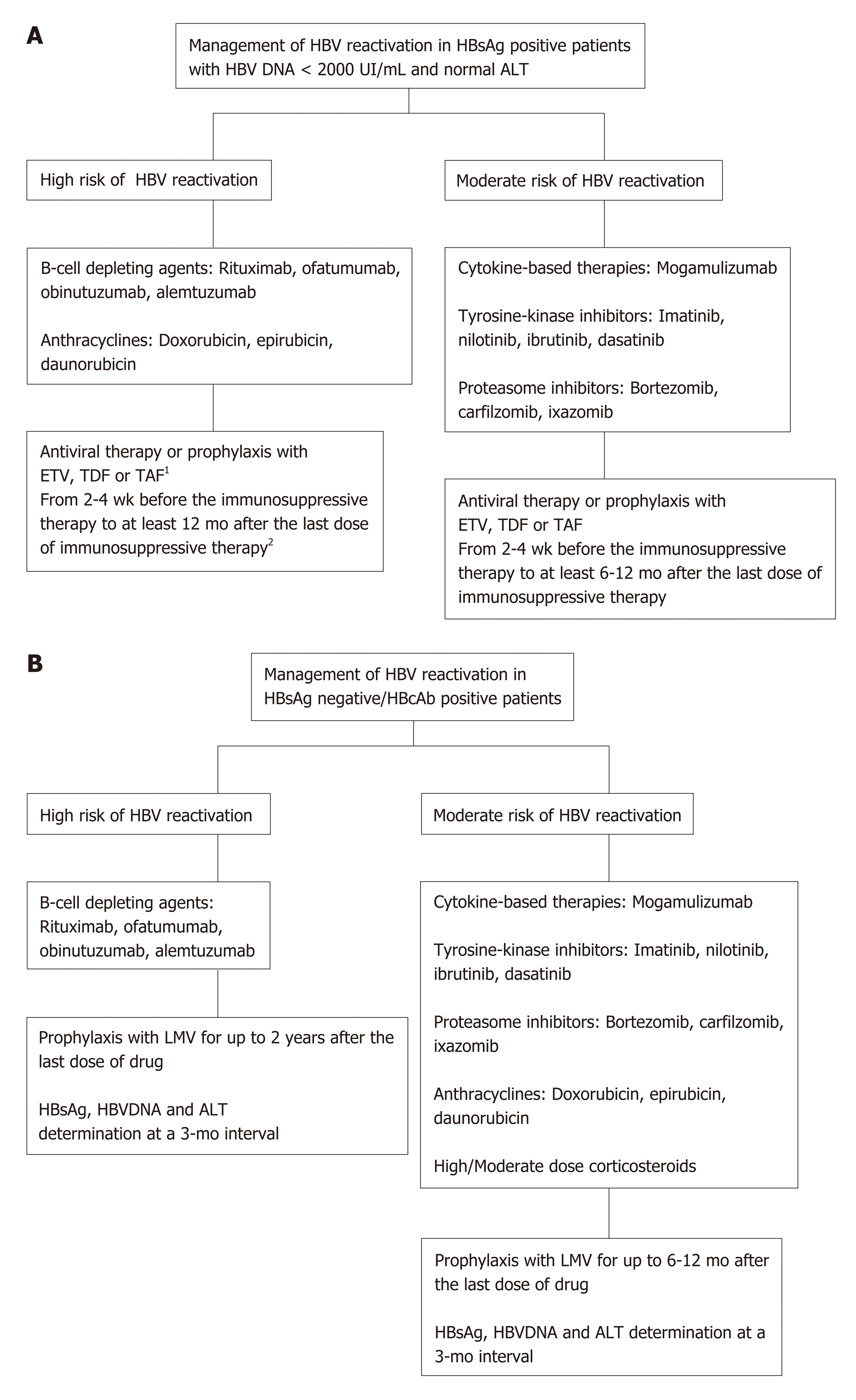
Figure 1 Management of hepatitis B virus reactivation.
A: Management of hepatitis B virus (HBV) reactivation in hepatitis B surface antigen (HBsAg) positive patients with HBV DNA < 2000 UI/mL and normal alanine aminotransferase (ALT); B: Management of HBV reactivation in HBsAg negative/hepatitis B core antibodies positive patients. 1HBsAg, HBV DNA and ALT determination at a 3-mo interval; 2In patients treated with rituximab or other cell-depleting agents, the anti-HBV prophylaxis should be continued for up to 2 years after the last dose of drug. HBV: Hepatitis B virus; HBVr: Hepatitis B virus replication; HbsAg: Hepatitis B surface antigen; HbcAb: Hepatitis B core antibodies; ETV: Entecavir; TDF: Tenofovir; TAF: Tenofovir alafenamide; LMV: Lamivudine; ALT: Alanine aminotransferase.
- Citation: Sagnelli C, Pisaturo M, Calò F, Martini S, Sagnelli E, Coppola N. Reactivation of hepatitis B virus infection in patients with hemo-lymphoproliferative diseases, and its prevention. World J Gastroenterol 2019; 25(26): 3299-3312
- URL: https://www.wjgnet.com/1007-9327/full/v25/i26/3299.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i26.3299