Copyright
©The Author(s) 2019.
World J Gastroenterol. Mar 14, 2019; 25(10): 1266-1277
Published online Mar 14, 2019. doi: 10.3748/wjg.v25.i10.1266
Published online Mar 14, 2019. doi: 10.3748/wjg.v25.i10.1266
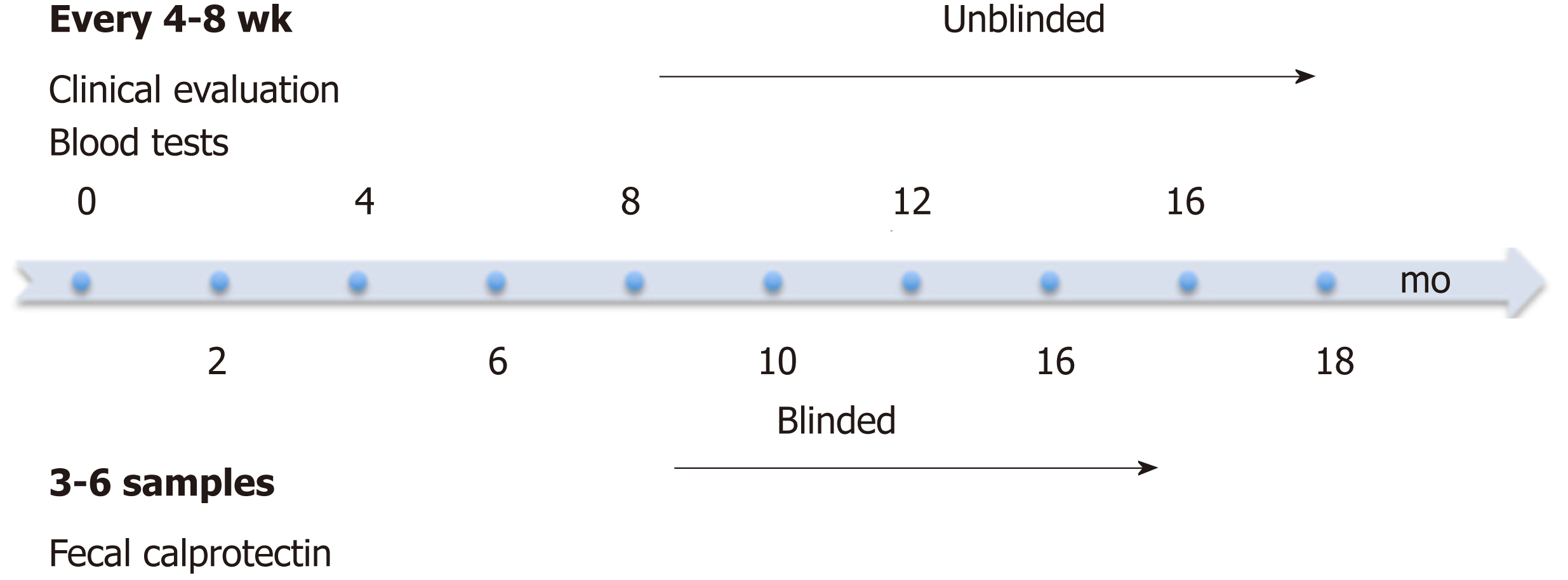
Figure 1 Study design.
Patients were clinically evaluated at study entry and then every 4-8 wk at infusion visits. Blood tests for CRP, ESR and albumin were performed at each infusion visit. FC levels were measured every 4-8 wk for the first 3-6 visits. FC: Fecal calprotectin; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein.
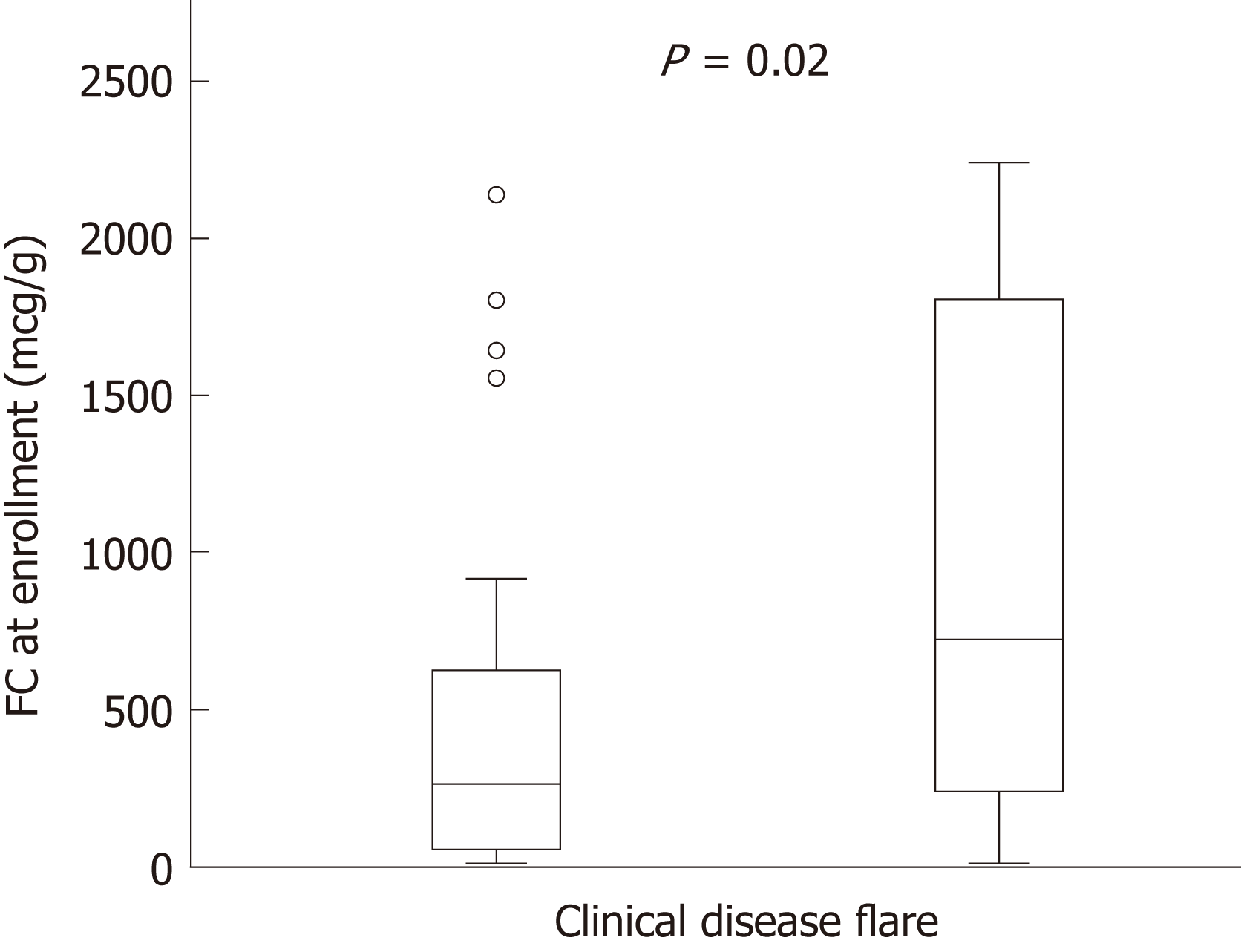
Figure 2 Increased baseline fecal calprotectin values in pediatric patients with inflammatory bowel disease are associated with elevated risk for clinical relapse.
P = 0.02. Bar graphs represent median values with interquartile range. FC: Fecal calprotectin.
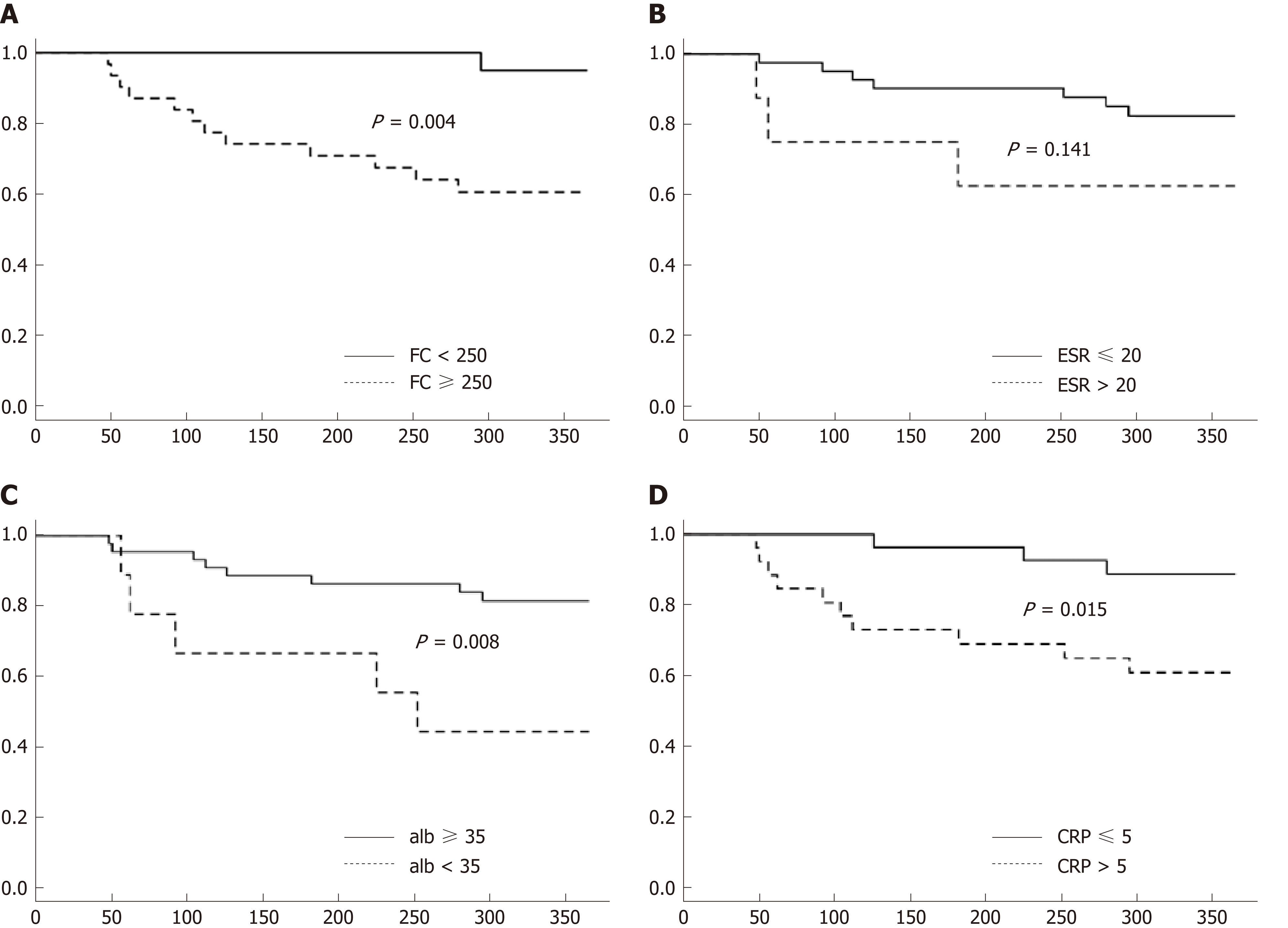
Figure 3 Kaplan-Meier curves at baseline through the first year of follow-up showing differences in predicting clinical relapse.
A: Kaplan-Meier curves for FC; B: Kaplan-Meier curves for ESR; C: Kaplan-Meier curves for CRP; D: Kaplan-Meier curves for albumin. FC: Fecal calprotectin; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein.
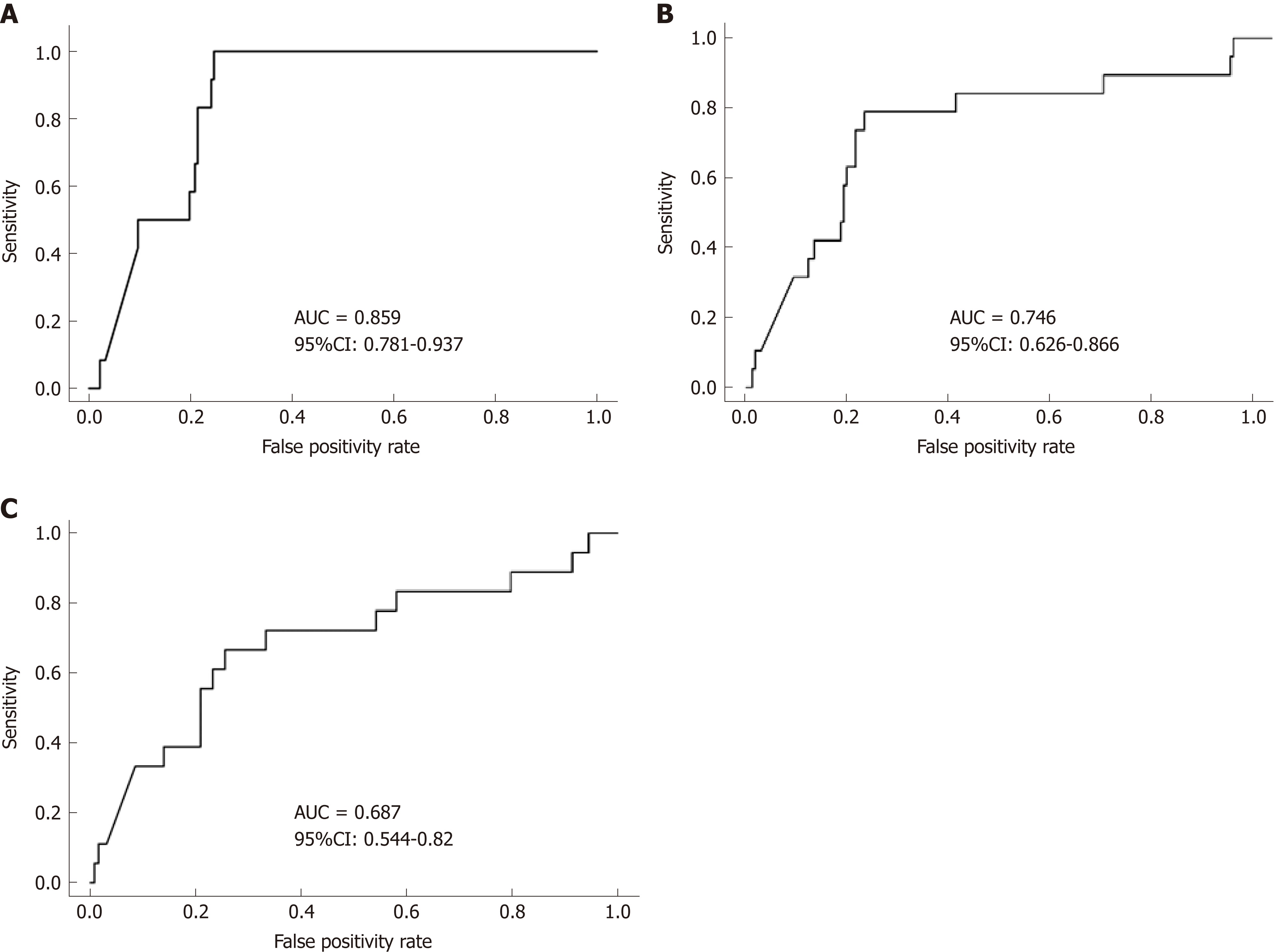
Figure 4 Receiver operator characteristic curve analysis for predicting clinical disease relapse.
A: 3-mo; B: 6-mo; C: 12-mo. FC levels at the 3-mo visit more accurately predict clinical disease relapse than at 6-mo and 12-mo post baseline (univariate analysis). FC: Fecal calprotectin; AUC: Area under the curve; CI: Confidence interval.
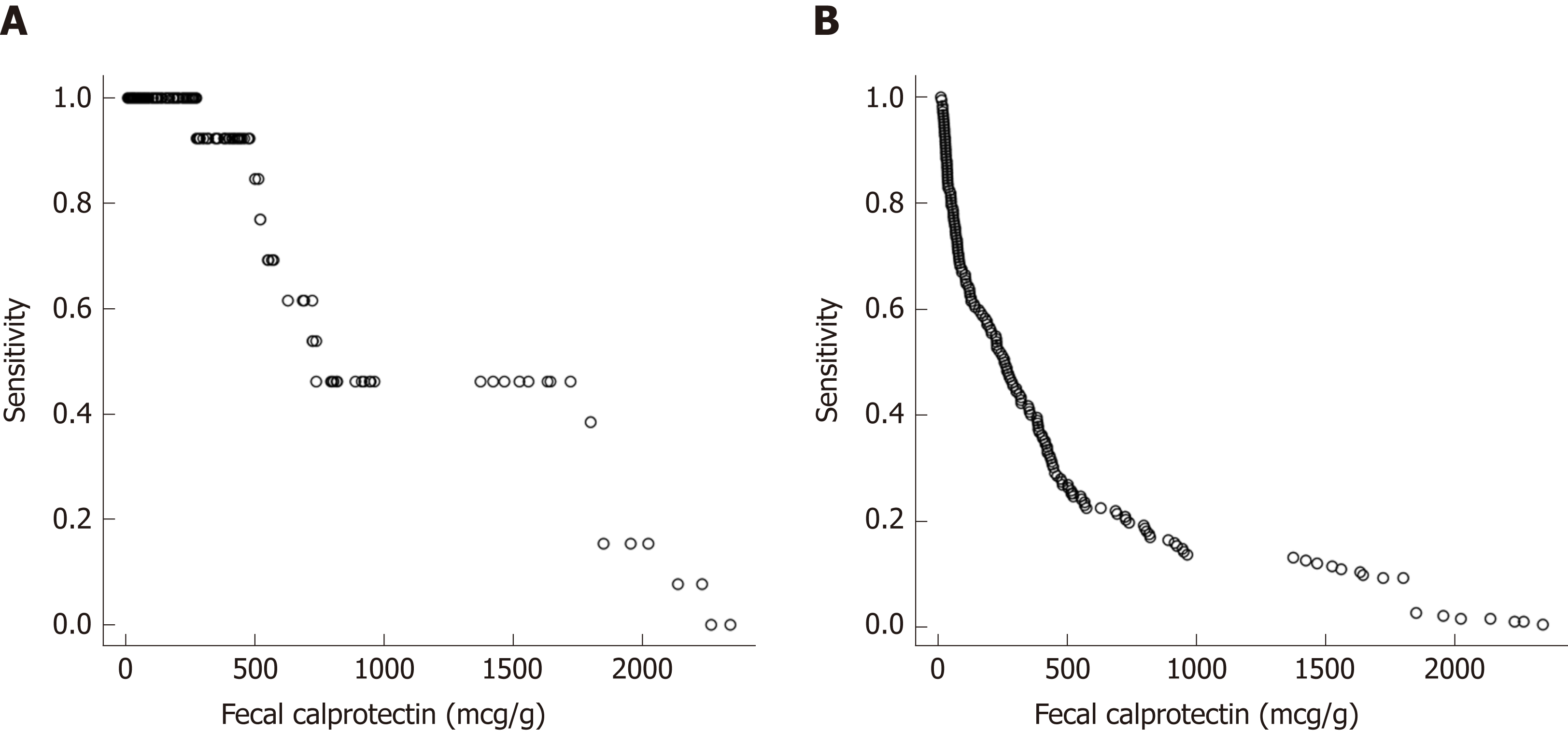
Figure 5 Sensitivity at various fecal calprotectin cut off values to predict a clinical disease flare.
A: Sensitivity at various fecal calprotectin cut off values to predict a clinical disease flare at the next visit; B: False positive rates to predict a disease flare at the next visit for various FC levels (repeated measures). FC: Fecal calprotectin.
- Citation: Foster AJ, Smyth M, Lakhani A, Jung B, Brant RF, Jacobson K. Consecutive fecal calprotectin measurements for predicting relapse in pediatric Crohn’s disease patients. World J Gastroenterol 2019; 25(10): 1266-1277
- URL: https://www.wjgnet.com/1007-9327/full/v25/i10/1266.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i10.1266