Copyright
©The Author(s) 2018.
World J Gastroenterol. Dec 28, 2018; 24(48): 5454-5461
Published online Dec 28, 2018. doi: 10.3748/wjg.v24.i48.5454
Published online Dec 28, 2018. doi: 10.3748/wjg.v24.i48.5454
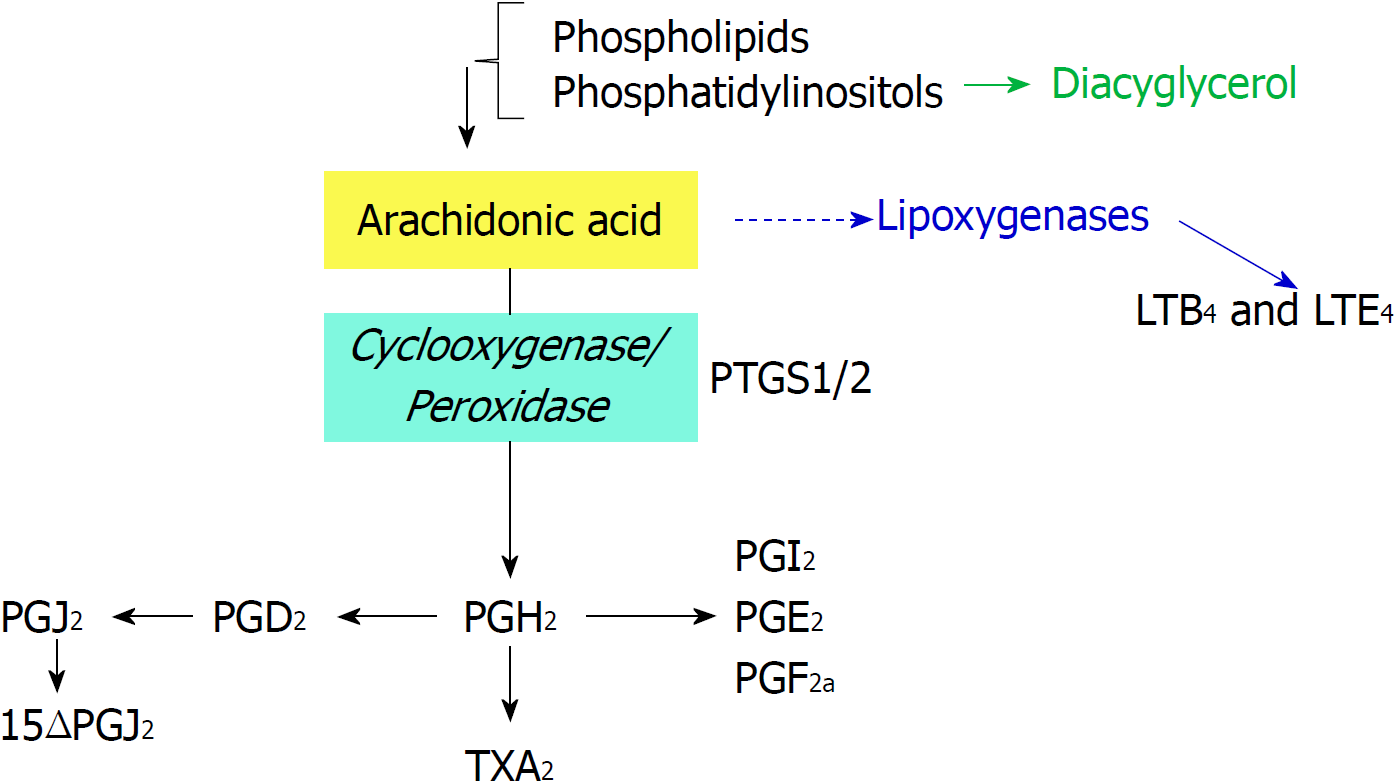
Figure 1 Biochemical reactions catalyzed by prostaglandin-endoperoxide synthase using arachidonic acid as substrate.
Phospholipids are cleaved by phospholipases to render arachidonic acid. Prostaglandin-endoperoxide synthase activity generates the precursor prostaglandin H2 that is converted in the different prostaglandins (PGs) by prostaglandin synthases and thromboxane A2, by thromboxane synthase. PG: Prostaglandin; PTGS: Prostaglandin-endoperoxide synthase.
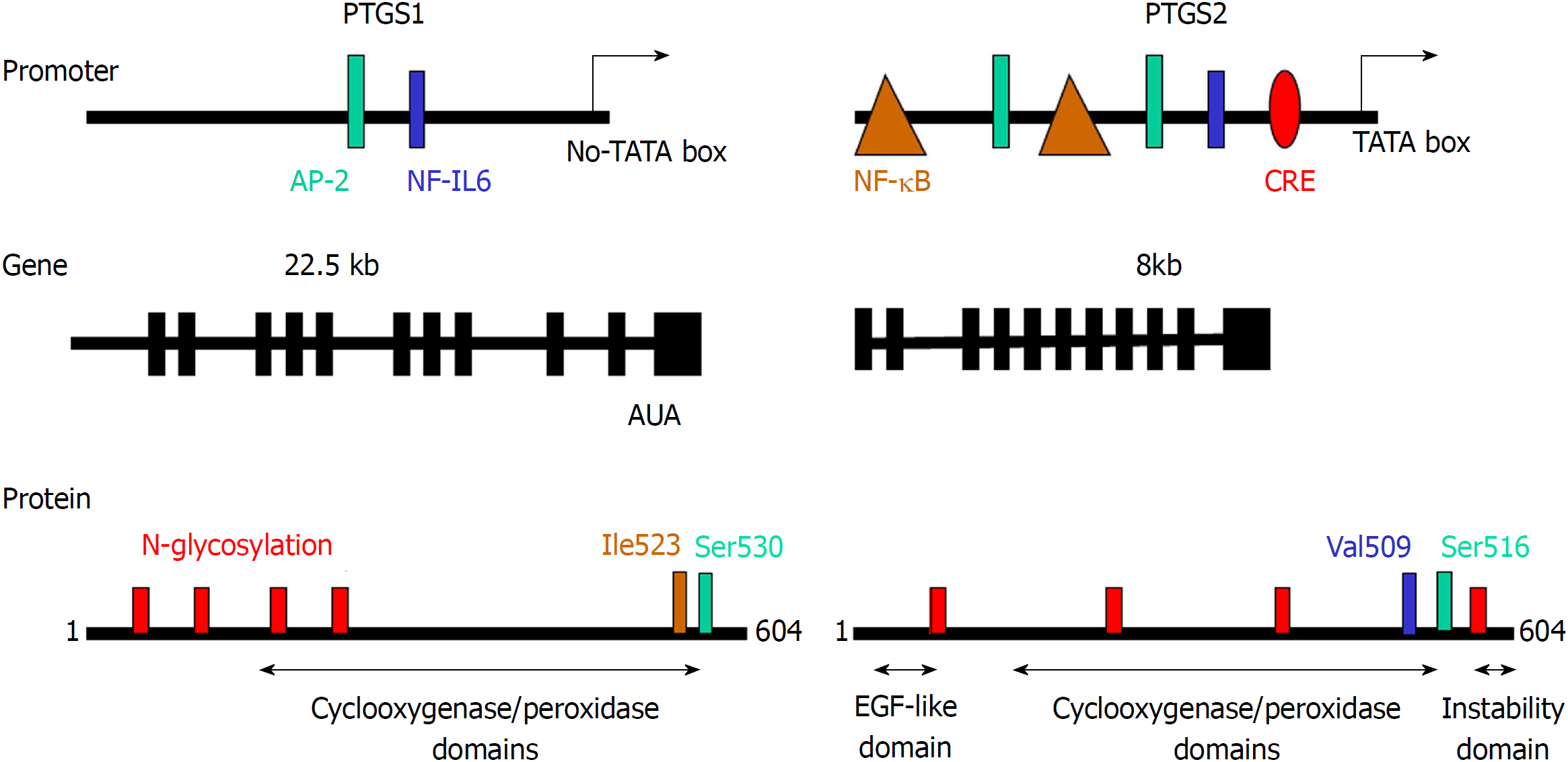
Figure 2 Comparison of the promoter region, gene structure and protein sequence of consensus prostaglandin-endoperoxide synthase 1 and 2.
The scheme shows the main transcription factors involved in the transcriptional control of prostaglandin-endoperoxide synthase (PTGS), the structure of the mRNA and the structural motifs present in the protein and relevant for the activity of the enzyme. The glycosylation of PTGS has effects on the activity and fate of the protein, protecting PTGS2 from proteasomal degradation. In addition to this, the electrophoretic mobility of the protein is altered by the glycosylation status of the protein. PTGS: Prostaglandin-endoperoxide synthase; AP-2: Activating protein 2; CRE: cAMP response element; NF-κB: Nuclear factor kappa B.
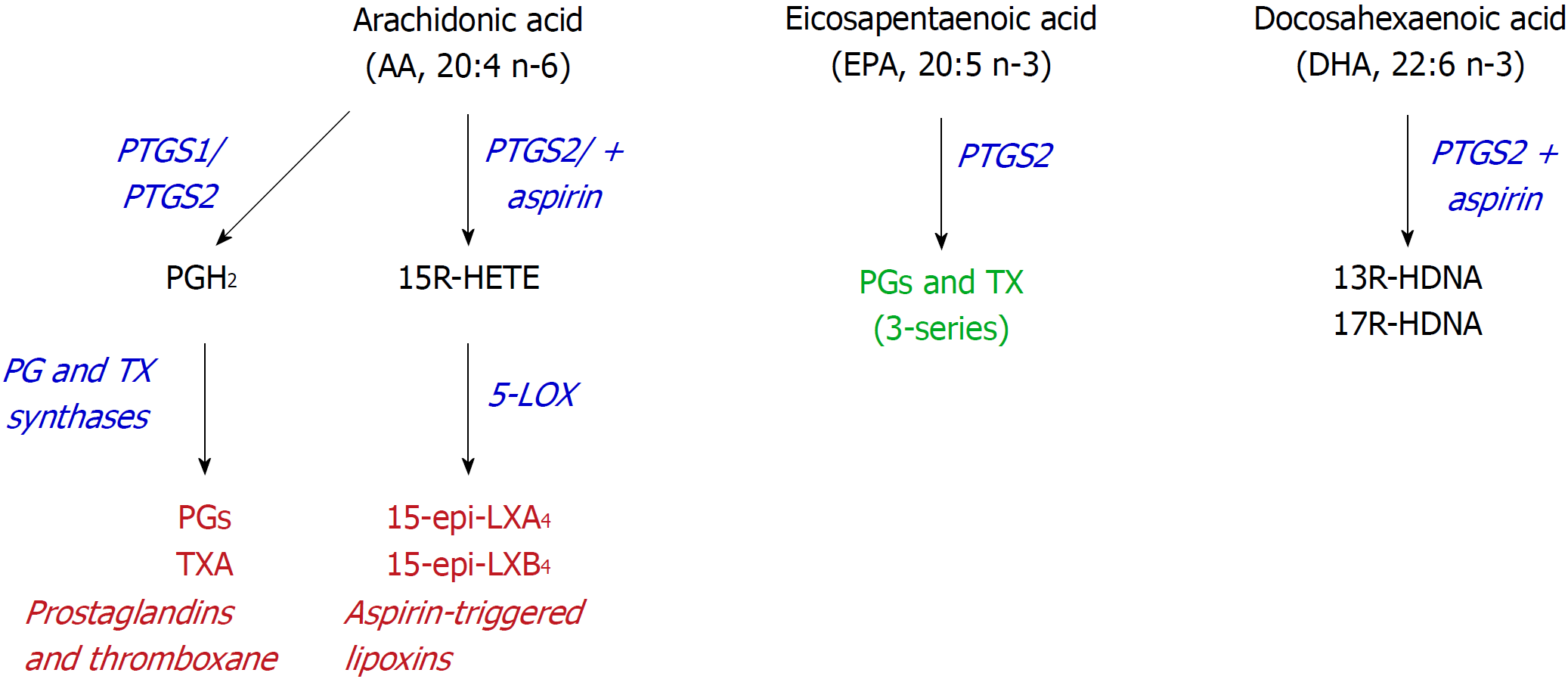
Figure 3 Alternative use of polyunsaturated fatty acids.
Aspirin may alter the activity of prostaglandin-endoperoxide synthase (PTGS) since it inhibits PTGS1, but retains the cyclooxygenase activity from PTGS2, leading to the synthesis of 15R-hydroxyeicosatetraenoic acid, from arachidonic acid, or 13- or 17R-hydroxy-docosahexaenoic acid, from docosahexaenoic acid. PTGS: Prostaglandin-endoperoxide synthase; HETE: Hydroxyeicosatetraenoic acid; HDHA: Hydroxy-docosahexaenoic acid.
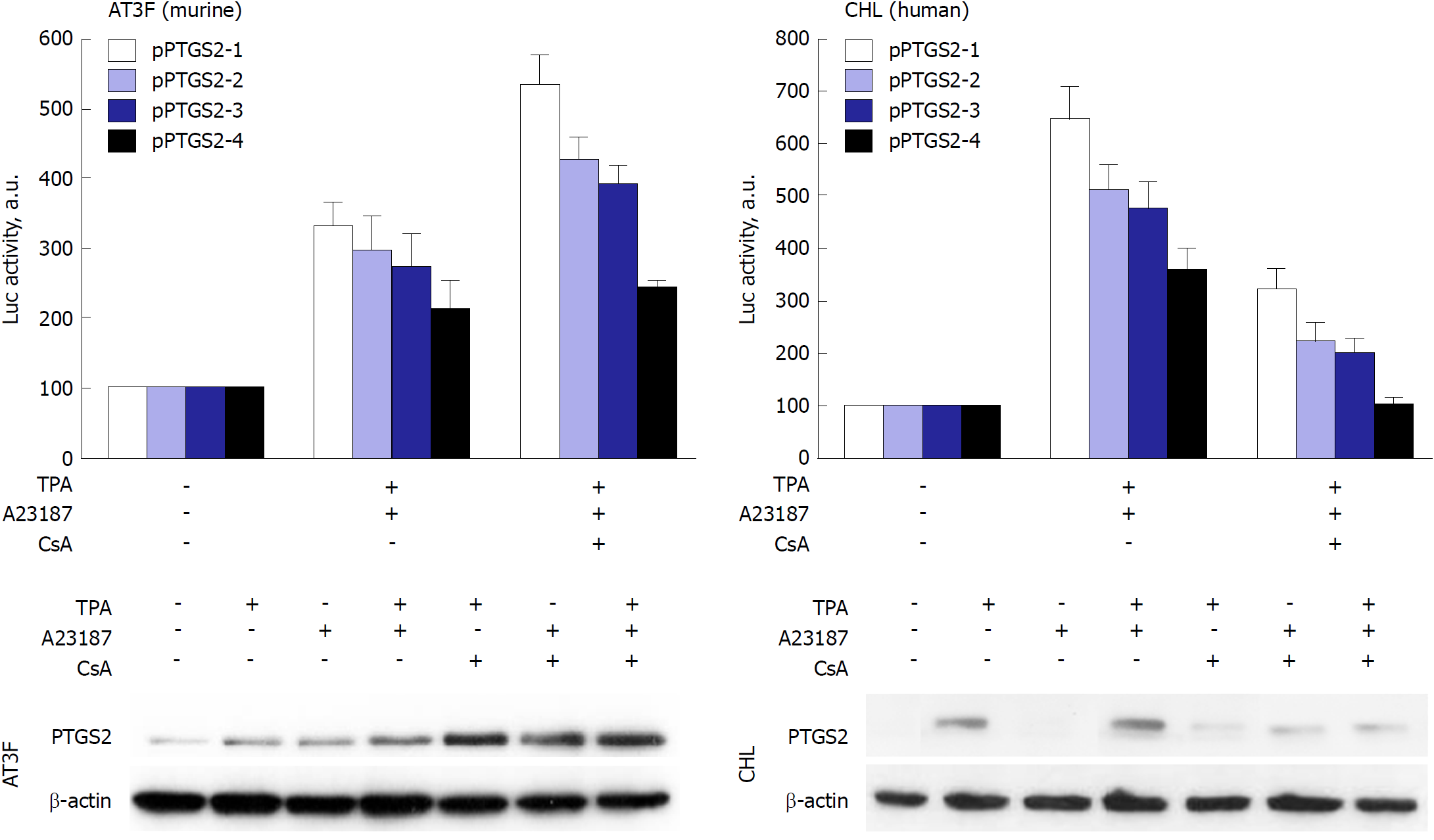
Figure 4 Prostaglandin-endoperoxide synthase 2 exhibits species-specific transcriptional control.
Despite the broad conservation of the transcription factor motifs in the prostaglandin-endoperoxide synthase 2 promoter, the activity of the promoter in response to the NF-AT inhibitor cyclosporine A (CsA) is repressed in human hepatic cell lines, but enhanced in murine hepatic counterparts. To confirm this effect, murine AT3F hepatic cells and human CHL hepatic cells were transfected with different constructions of the promoter linked to a luciferase reporter gene (see[46] for details). The protein levels and the luciferase activity were determined at 24h after treatment with the indicated stimuli: tetradecanoylphorbol acetate 100 nmol/L, Ca2+-ionophore A23187 1 μmol/L; CsA 100 nmol/L. TPA: Tetradecanoylphorbol acetate; CsA: Cyclosporine A; PTGS: Prostaglandin-endoperoxide synthase.
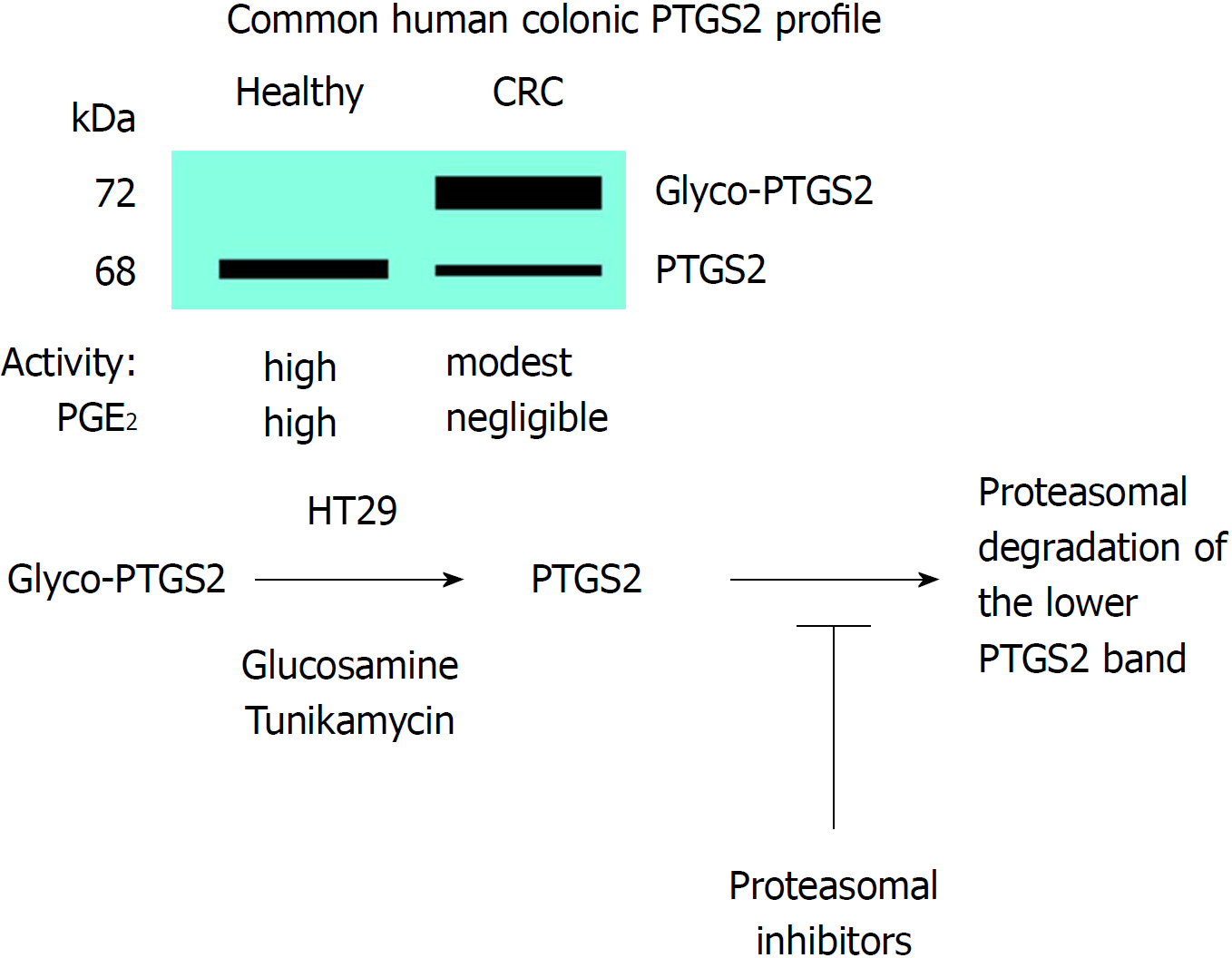
Figure 5 Glycosylation of prostaglandin-endoperoxide synthase 2 in colorectal cancer.
The main form in colorectal cancer tissues is the glycosylated 72 kDa protein. This glycosylated prostaglandin-endoperoxide synthase 2 (PTGS2) is also expressed in several tumor colonic cell lines and can be deglycosylated after incubation of the cells with glucosamine or tunikamycin. After this treatment, PTGS2 is rapidly degraded via proteasomal activity. CRC: Colorectal cancer; PTGS: Prostaglandin-endoperoxide synthase.
- Citation: Jaén RI, Prieto P, Casado M, Martín-Sanz P, Boscá L. Post-translational modifications of prostaglandin-endoperoxide synthase 2 in colorectal cancer: An update. World J Gastroenterol 2018; 24(48): 5454-5461
- URL: https://www.wjgnet.com/1007-9327/full/v24/i48/5454.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i48.5454