Copyright
©The Author(s) 2018.
World J Gastroenterol. May 28, 2018; 24(20): 2137-2151
Published online May 28, 2018. doi: 10.3748/wjg.v24.i20.2137
Published online May 28, 2018. doi: 10.3748/wjg.v24.i20.2137
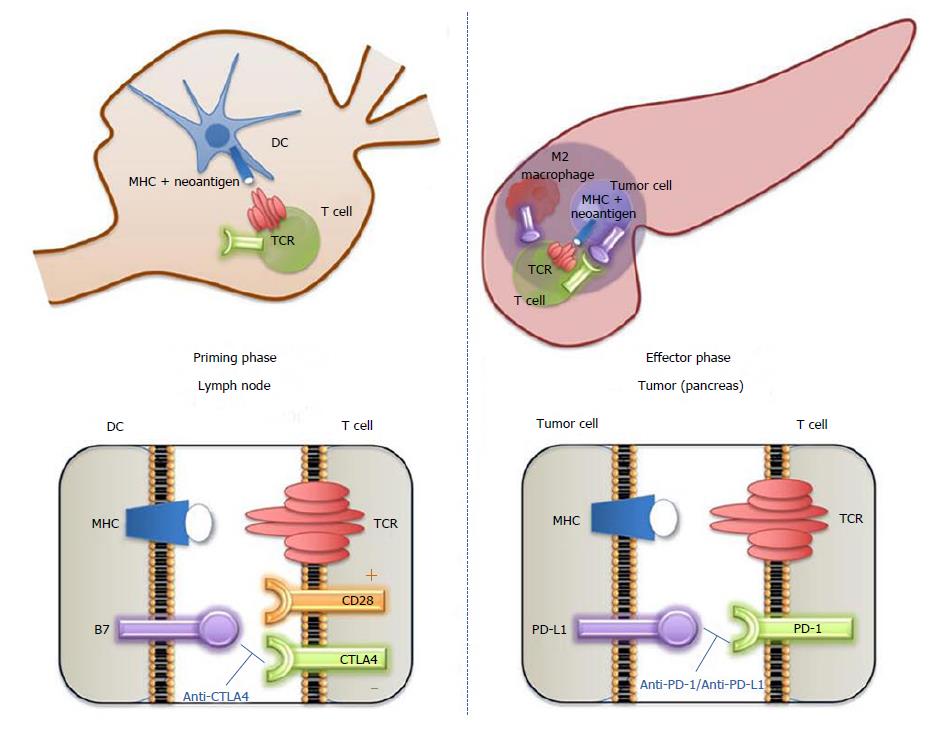
Figure 1 Cytotoxic T lymphocyte-associated protein 4 and programmed cell death-1 biological functions and therapeutic targeting.
Cells of the immune system express several surface molecules that are important for immune surveillance and regulation of the immune response. T cell receptor (TCR) is expressed by T cells; it is an antigen-specific molecule that is unique to each T cell clone. Major human compatibility (MHC) molecule is expressed by antigen-presenting cells (e.g., dendritic cell) and display a potential tumor antigen for recognition by the specific TCR. Left panel: When an antigen presented in the context of MHC is recognized by the TCR, interaction of CD28 (expressed by T cell) with B7 (CD80/CD86) molecules provide a co-stimulatory signal leading to T-cell activation. However, depending on the conditions and microenvironment, these T cells can also express various levels of cytotoxic T lymphocyte-associated protein 4 (CTLA-4), a regulatory receptor (immune checkpoint) with a higher binding affinity for B7 than CD28. Therefore, when CTLA-4 is available at the cell surface, it successfully competes for binding with B7, removing the co-stimulatory signal and leading to T-cell downregulation. Tumor cells can then escape the T cell cytotoxic effect (immune evasion). CTLA-4 blockade affects the immune priming phase occurring in the lymph node, by supporting the activation and proliferation of a higher number of effector T cells, regardless of TCR specificity, and by reducing Treg-mediated suppression of T-cell responses. Right panel: T cells also express PD-1 receptor, which has the potential to induce a programmed-death cascade in T cells that mistakenly react to host cells and thereby maintaining self-tolerance. PD-1 ligand, PD-L1, is used by tumor cells to engage the PD-1 receptor and switch off the reaction, inducing immune tolerance to the MHC-presented antigen. PD-L1 can also be expressed by stromal cells (e.g., M2 macrophages). PD-1 blockade works during the effector phase in peripheral tissues (tumor) to restore the immune function of “exhausted” T cells that have been turned off following extended or high levels of antigen exposure. CTLA-4: Cytotoxic T lymphocyte-associated protein 4; DC: Dendritic cell; MHC: Major human compatibility; PD-1: Programmed cell death-1; PD-L1: Programmed death-ligand 1; TCR: T cell receptor.
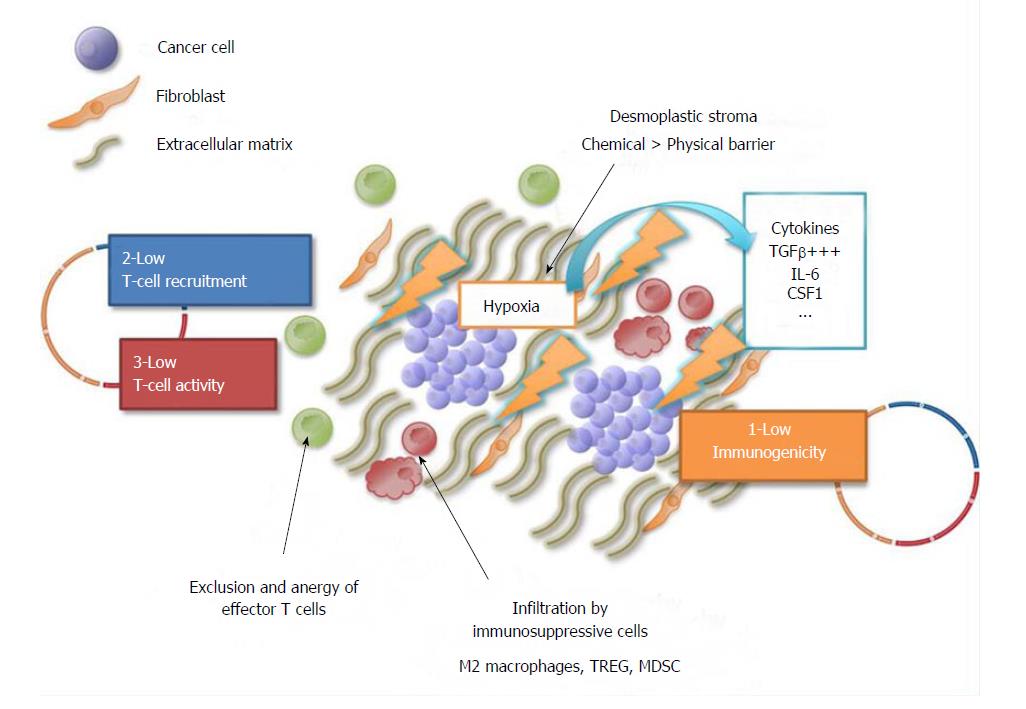
Figure 2 Summary of the mechanisms responsible for pancreatic ductal adenocarcinoma resistance to immune therapy.
The circle outlines the three steps of the cancer-immunity cycle: (1) Immunogenicity (yellow); (2) T-cell recruitment and (3) activation. Pancreatic ductal adenocarcinoma resistance to immune therapy is due to the combination of several factors: (1) Low tumor immunogenicity, with a low mutation rate and low neaoantigen burden compared to other tumors (e.g., melanoma); (2) low T-cell recruitment and (3) activation: the dense desmoplastic stroma generates high interstitial pressure; this results in poor tumor perfusion and intra-tumor hypoxia, which in turn activates fibroblasts to release immunosuppressive cytokines (e.g., TGFβ, IL-6, CSF1 = “chemical barrier”) that lead to the recruitment of immunosuppressive cells (M2 macrophages, TREG, MDSC) and exclusion and anergy of effector T cells. CSF1: Colony stimulating factor 1; IL-6: Interleukin-6; MDSC: Myeloid-derived suppressive cells; TGFβ: Transforming growth factor β; TREG: T regulatory cells.
- Citation: Hilmi M, Bartholin L, Neuzillet C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J Gastroenterol 2018; 24(20): 2137-2151
- URL: https://www.wjgnet.com/1007-9327/full/v24/i20/2137.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i20.2137