Copyright
©The Author(s) 2018.
World J Gastroenterol. Apr 7, 2018; 24(13): 1373-1385
Published online Apr 7, 2018. doi: 10.3748/wjg.v24.i13.1373
Published online Apr 7, 2018. doi: 10.3748/wjg.v24.i13.1373
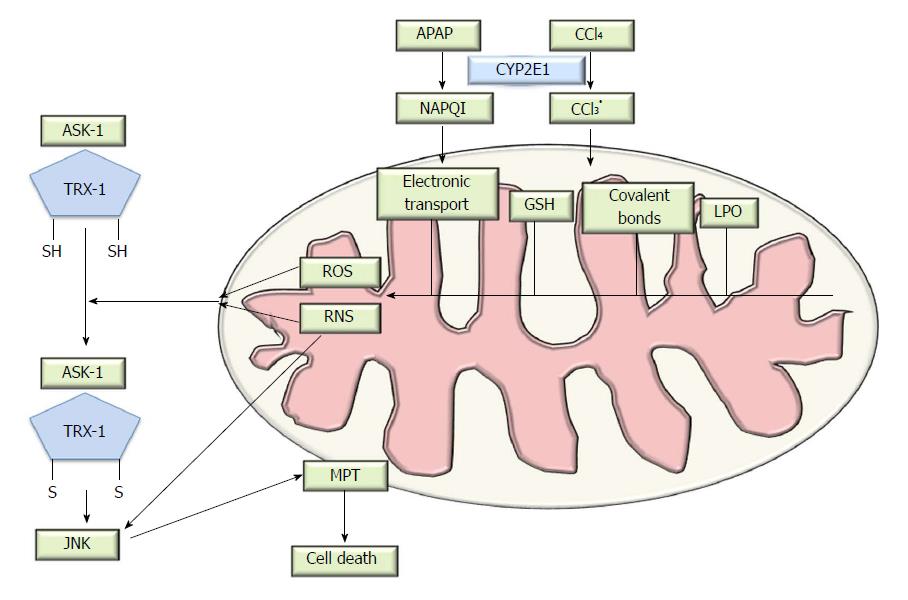
Figure 1 Pathophysiology of drug-induced liver injury.
Schematic representation of paracetamol toxicology. Metabolism of acetaminophen (APAP) or carbon tetrachloride (CCl4) catalyzed by CYP2E1 enzyme causes the generation of an intermediate reactive compound which causes covalent bonds, glutathione (GSH) depletion and increased in oxidative stress. Thioredoxin-1 (Trx-1) normally binds the N-terminal domain of Apoptosis signal-regulating kinase 1 (ASK1) and inhibits kinase activity. Reactive oxygen species (ROS) accumulation oxidizes and consequently removes Trx-1 from Trx-ASK1 complexes, leading to activation of ASK1 and subsequent apoptosis signalling cascade. Then c-Jun N-terminal kinases (JNK) translocates into the mitochondria and alters of the mitochondrial membrane potential, which triggers cell death. DILI: Drug-induced liver injury. MPT: Membrane permeability transition; LPO: Lipid peroxidation; NAPQI: N-acetyl-p-benzoquinone imine.
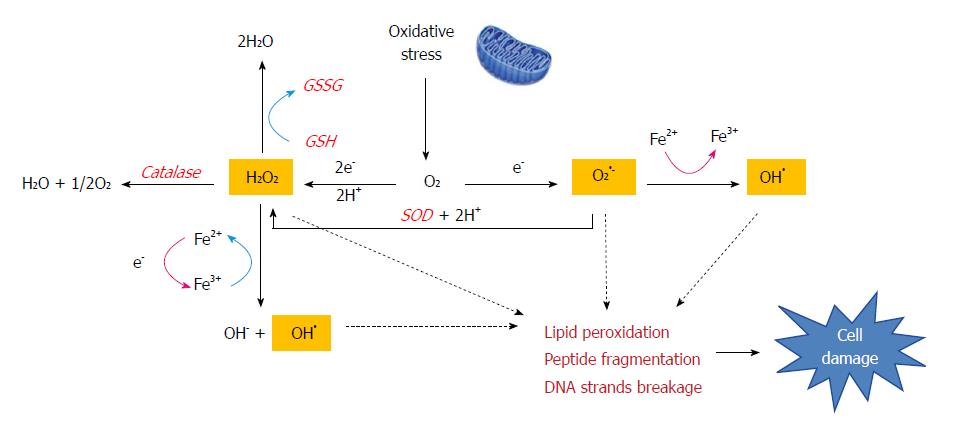
Figure 2 The Fenton reaction in liver disease.
Oxidative stress produces large amounts of reactive compounds and cytotoxic free radicals (H2O2, O2•- and OH•). The Fenton reaction generates hydroxyl radicals (OH•) from hydrogen peroxide (H2O2) and superoxide (O2•-) catalyzed by iron. This reaction occurs in cells and free radicals can attack the double bonds of non-saturated phospholipids in cell membranes which eventually degrade the structural integrity of cell membranes, impair enzymatic function and cause cross-linking of proteins or strand breaks in DNA. Cells also have an antioxidant enzyme system (catalase, GSH or SOD) is meant to neutralize free radicals and prevent damage.
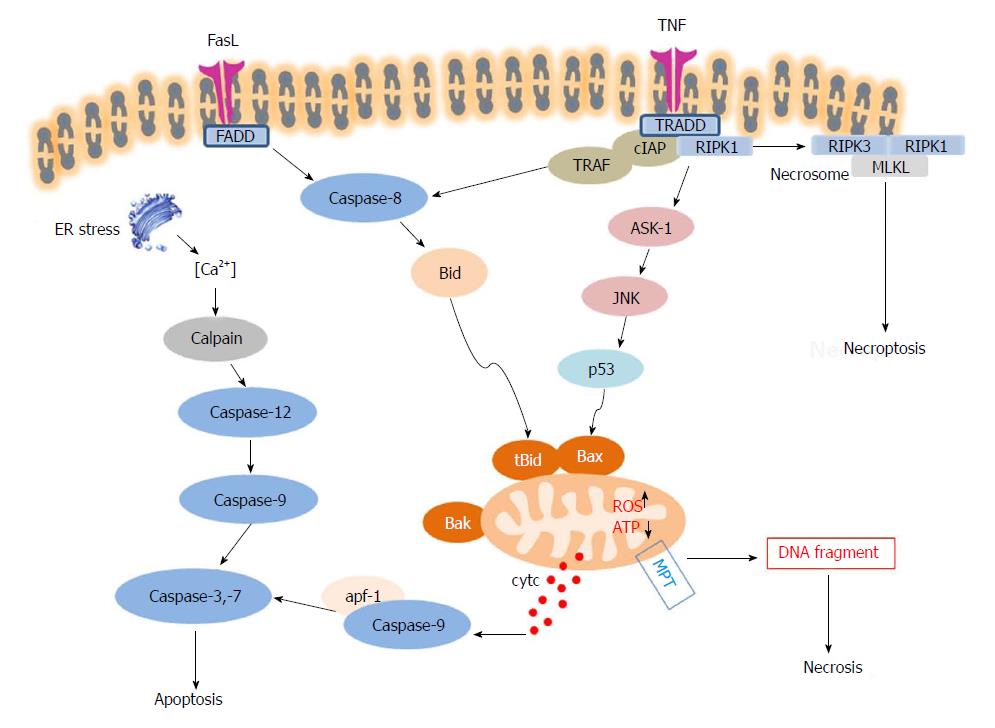
Figure 3 Schematic overview of three different modes of cell death: apoptosis, necrosis and necroptosis.
When FasL or Tumor necrosis factor-alpha (TNF-α) bind to their death receptors (DRs), death domains of DRs are oligomerized and form death-inducing signalling complex (DISC), which recruits caspase-8. Active caspase 8 cleaves Bid into cleaved Bid (tBid), which translocates to mitochondria and cooperates with Bax. Meanwhile, JNKs are activated by Mitogen-activated protein kinases (MAPKs) and pJNK also translocates to the mitochondria via binding to the Sab protein. ROS accumulation and ATP depletion in the mitochondria aggravate mitochondrial damage and induce membrane permeability transition (MPT), resulting in release of cytochrome C, in turn promoting the activation of caspase-9 and caspase-3. Activated caspase-3 then leads to hepatocyte apoptosis. The extrinsic and intrinsic pathways of hepatocyte apoptosis are linked, because hepatocytes require mitochondrial amplification activating caspase-3 for cell death execution. The mitochondrial injury and MPT are also key factors in cascade of events leading to necrosis. Necroptosis shares the upstream pathway with apoptosis. When cellular inhibitors of apoptosis (cIAPs) are depleted, Receptor interacting protein kinase (RIPK)1 and RIPK3 interact with each other via membrane permeability transition (RHIM) domains to form the necrosome, and further recruit and phosphorylate MLKL to initiate necroptosis.
- Citation: Ye H, Nelson LJ, Gómez del Moral M, Martínez-Naves E, Cubero FJ. Dissecting the molecular pathophysiology of drug-induced liver injury. World J Gastroenterol 2018; 24(13): 1373-1385
- URL: https://www.wjgnet.com/1007-9327/full/v24/i13/1373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i13.1373