Copyright
©The Author(s) 2017.
World J Gastroenterol. Feb 7, 2017; 23(5): 830-841
Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.830
Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.830
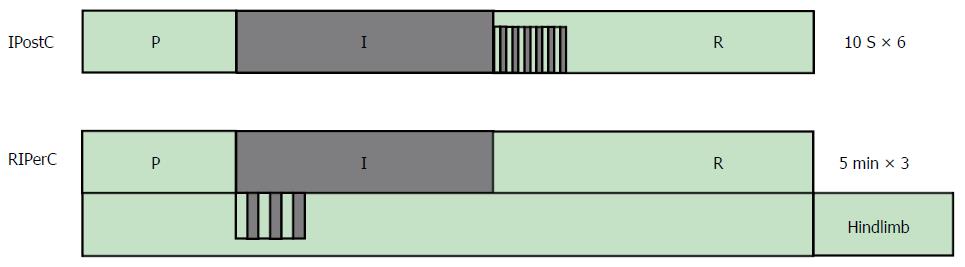
Figure 1 Ischemic postconditioning and remote ischemic perconditioning models.
IPostC was performed by six 10-s cycles of reperfusion and 10 s reocclusion of the portal vein. RIPerC was performed by three 5-min cycles of reperfusion and 5 min reocclusion by tourniquet. IPostC: Ischemic postconditioning; RIPerC: Remote ischemic perconditioning.
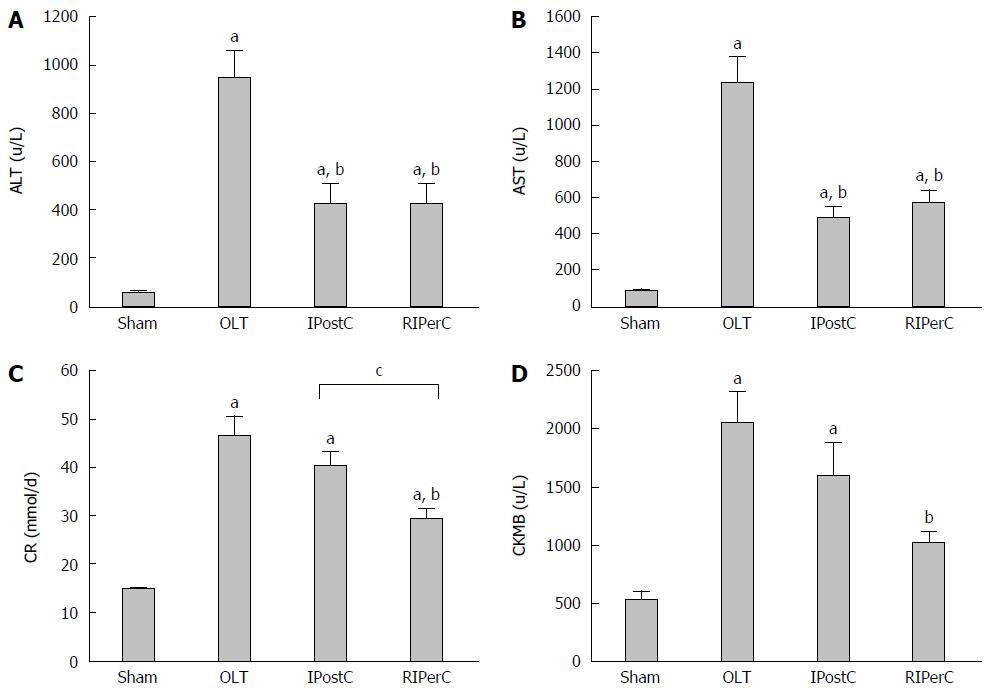
Figure 2 Effects of remote ischemic perconditioning treatment on liver graft and other organ functions.
A and B: Effects of remote ischemic perconditioning (RIPerC) and ischemic postconditioning (IPostC) on alanine aminotransferase (ALT) and aspartate aminotransferase (AST), parameters for hepatocellular damage; C: Effects of RIPerC and IPostC on creatinine (Cr), a marker of acute kidney injury; D: Effects of RIPerC and IPostC on creatinine kinase-myocardial band (CK-MB), a marker for acute myocardial damage. Data represent mean ± SEM for 5 animals per group. aP < 0.05 vs sham group; bP < 0.05 vs orthotopic liver transplantation (OLT) group; cP < 0.05 RIPerC vs IPostC.
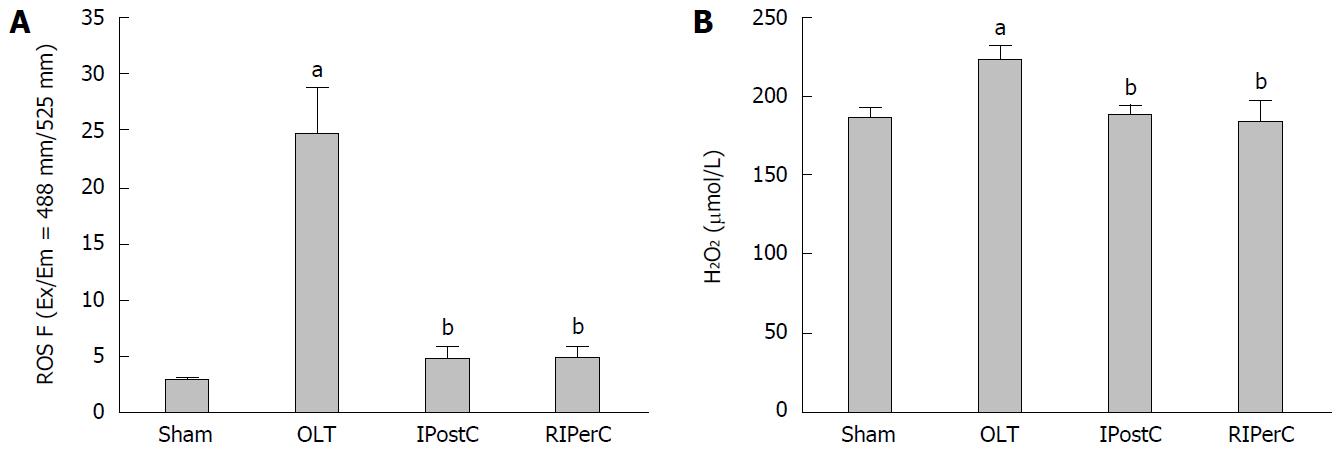
Figure 3 Effects of remote ischemic perconditioning on ischemia/reperfusion-induced oxidative stress.
A: Effects of remote ischemic perconditioning (RIPerC) and ischemic postconditioning (IPostC) on ROS expression; B: Effects of RIPerC and IPostC on H2O2 expression. Data represent mean ± SEM for 5 animals per group. aP < 0.05 vs sham group; bP < 0.05 vs orthotopic liver transplantation (OLT) group.
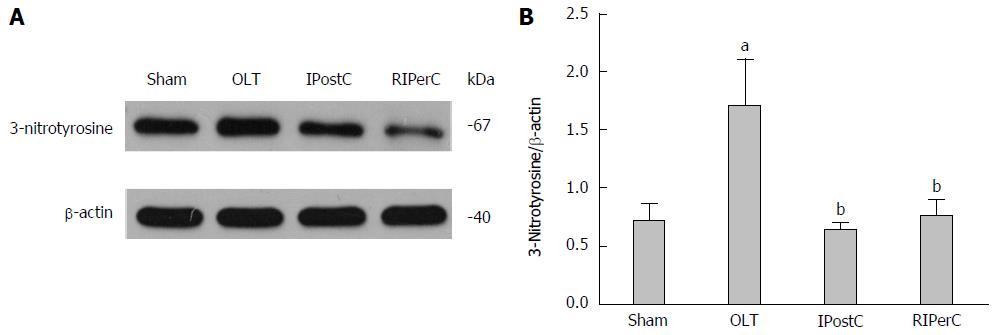
Figure 4 Effects of remote ischemic perconditioning on ischemia/reperfusion-induced nitrative stress.
A: Protein expression levels of 3-nitrotyrosine were measured by western blot analysis (the gels were run under the same experimental conditions); B: Statistical analysis of 3-nitrotyrosine in liver tissues is shown. Data represent mean ± SEM for 3 animals per group. aP < 0.05 vs sham group; bP < 0.05 vs orthotopic liver transplantation (OLT) group. RIPerC: Remote ischemic perconditioning.
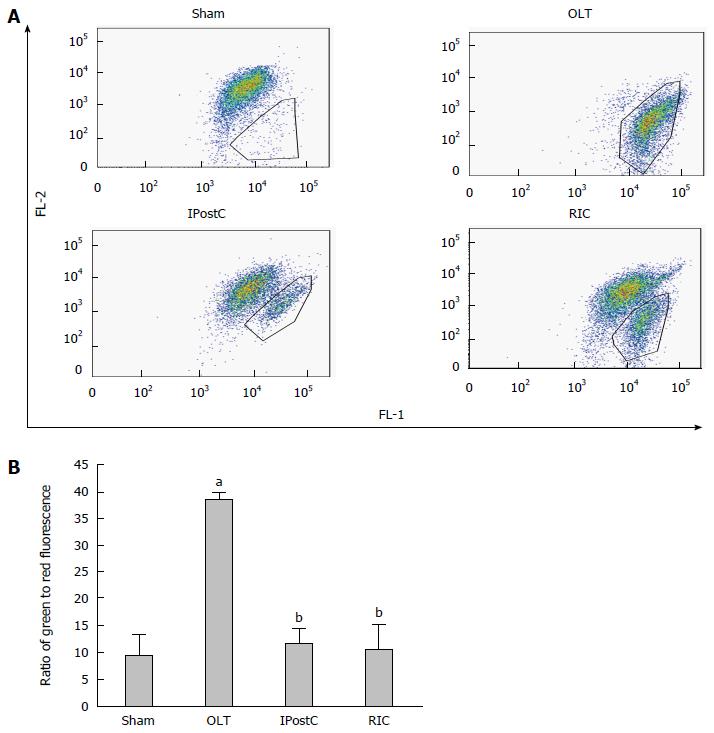
Figure 5 Effects of remote ischemic perconditioning on ΔΨm.
A: Typical dot plot analysis of JC-1 of Δm. A dot plot of red fluorescence (FL2) vs green fluorescence (FL1) resolved live liver cells with intact ΔΨm (Sham group) from apoptotic and dead cells with lost ΔΨm (OLT group); B: The change of Δm was reported by the ratio of green to red fluorescence. Data represent mean ± SEM for 5 animals per group. aP < 0.05 vs sham group; bP < 0.05 vs OLT group. OLT: Orthotopic liver transplantation.
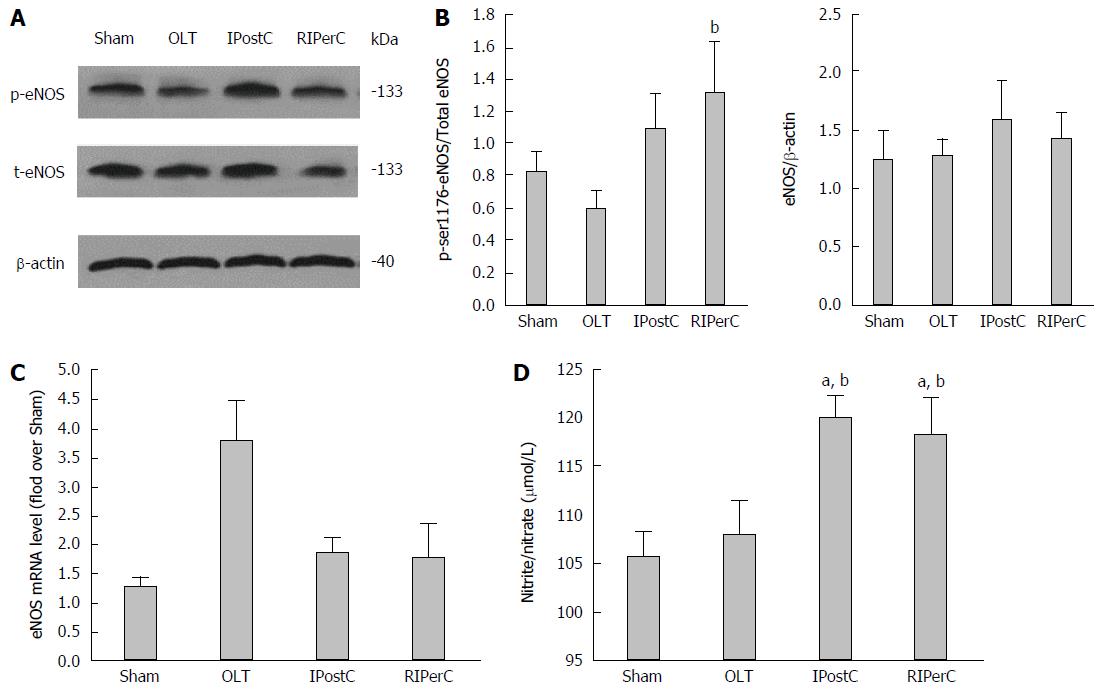
Figure 6 Effects of remote ischemic perconditioning on endothelial nitric oxide synthase and nitric oxide expression.
A: Western blots of phospho-ser1176-endothelial nitric oxide synthase (eNOS) [p-eNOS] and total eNOS (t-eNOS) detection in liver tissues (gels run under the same experimental conditions); B: Statistical analysis of phospho-ser1176-eNOS and total eNOS in liver tissues; C: mRNA level of eNOS; D: Total nitrate/nitrite content in the four groups. Data represent mean ± SEM for 5 animals per group. aP < 0.05 vs sham group; bP < 0.05 vs orthotopic liver transplantation (OLT) group.
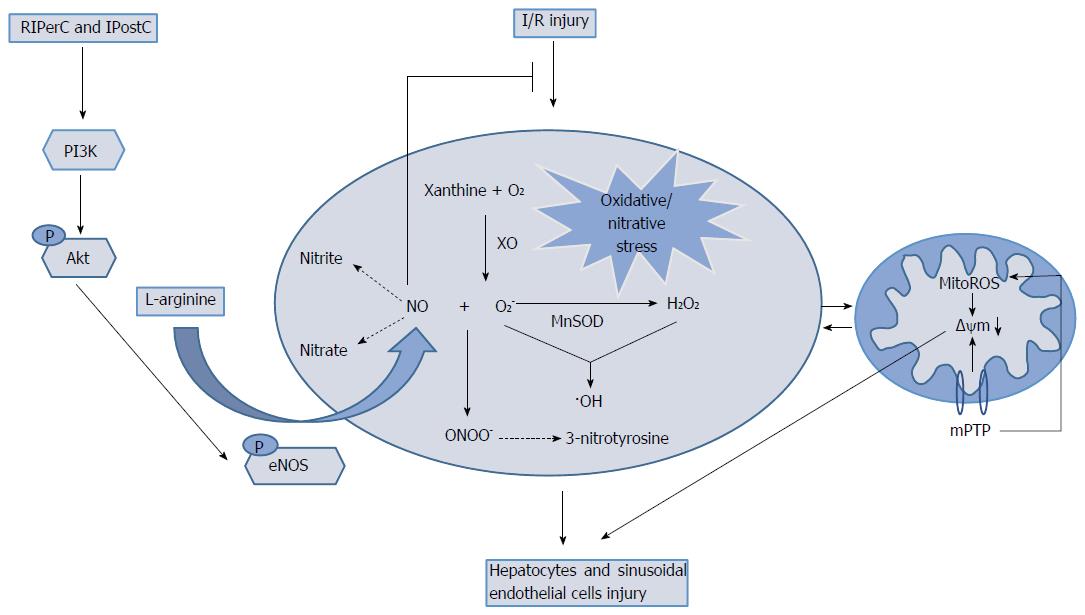
Figure 7 Simplified mechanisms of remote ischemic perconditioning protective effects against ischemia/reperfusion injury.
Remote ischemic perconditioning (RIPerC) liver graft protection involves inhibition of oxidative/nitrative stress and up-regulation of the PI3K/Akt/eNOS/NO pathway. •OH: Hydroxyl radical; XO: Xanthine oxidase; MitoROS: Mitochondrial reactive oxygen species. IPostC: Ischemic postconditioning; I/R: Ischemia/reperfusion.
- Citation: He N, Jia JJ, Li JH, Zhou YF, Lin BY, Peng YF, Chen JJ, Chen TC, Tong RL, Jiang L, Xie HY, Zhou L, Zheng SS. Remote ischemic perconditioning prevents liver transplantation-induced ischemia/reperfusion injury in rats: Role of ROS/RNS and eNOS. World J Gastroenterol 2017; 23(5): 830-841
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/830.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.830