Copyright
©The Author(s) 2017.
World J Gastroenterol. Nov 7, 2017; 23(41): 7478-7488
Published online Nov 7, 2017. doi: 10.3748/wjg.v23.i41.7478
Published online Nov 7, 2017. doi: 10.3748/wjg.v23.i41.7478
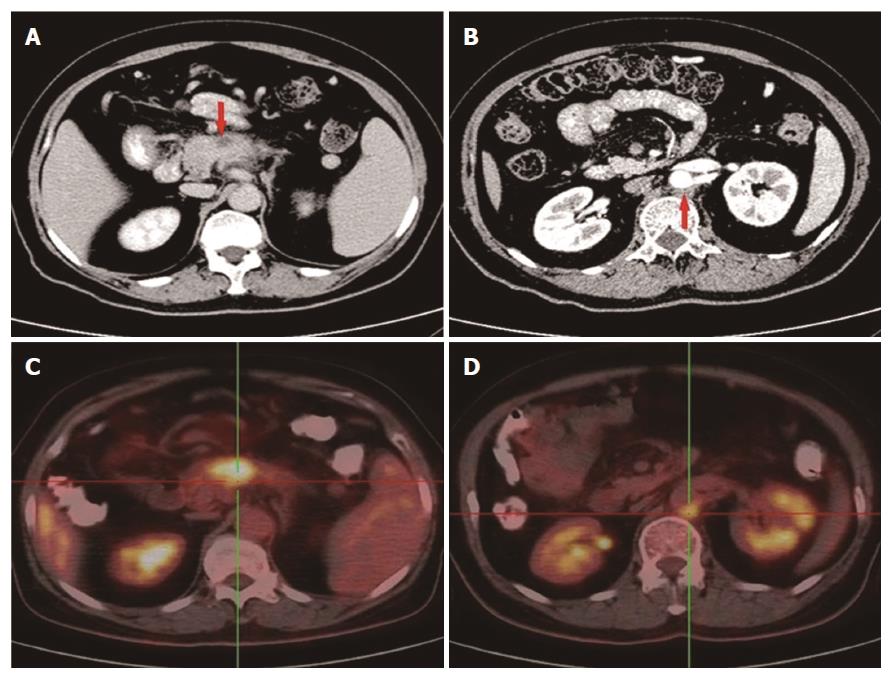
Figure 1 Abdominal CT and 18F-FDG PET/CT show lesions located in the pancreas and behind the aorta abdominalis.
A: A 3.1 cm × 1.7 cm mass at the body of the pancreas; B: An enlarged lymph node behind the aorta abdominalis; C: The mass in the body of the pancreas with an SUV of 6.2; D: The enlarged lymph node with an SUV of 4.8. CT: Computed tomography; PET: Positron emission tomography.
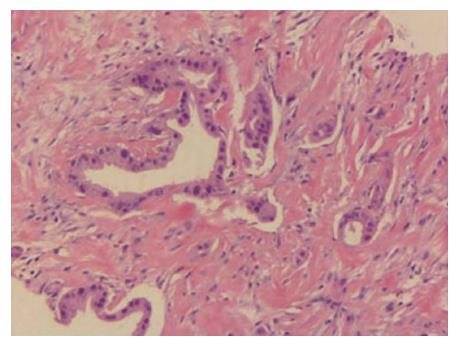
Figure 2 Hematoxylin and eosin staining of a tumor section (× 200).
The pathological diagnosis was moderately differentiated adenocarcinoma.
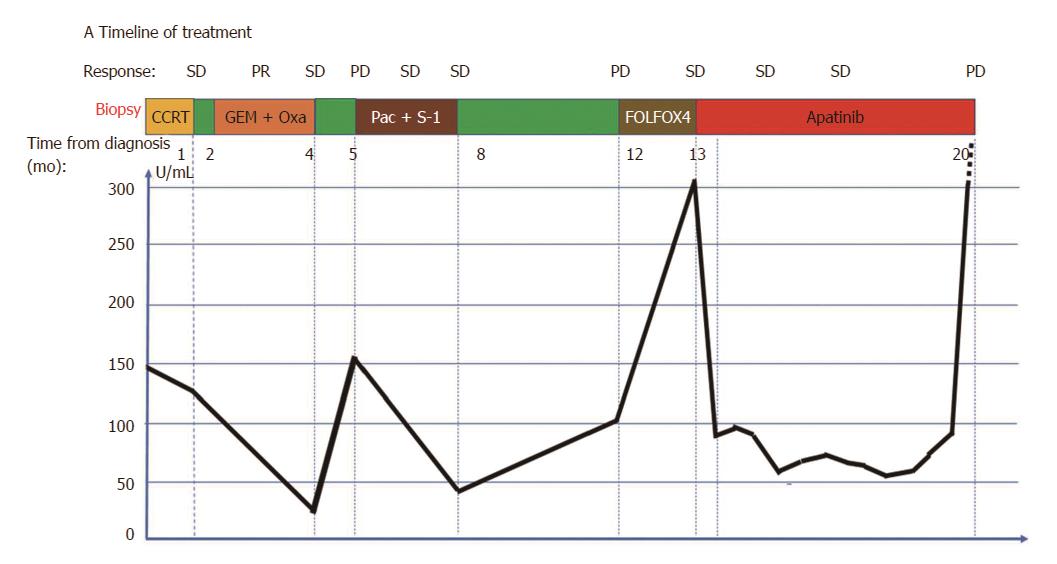
Figure 3 Timeline of treatment (A) and trend in carbohydrate antigen 19-9 level during treatment.
Oxa: Oxaliplatin; Pac: Paclitaxel-albumin. Green color indicates time without treatment.
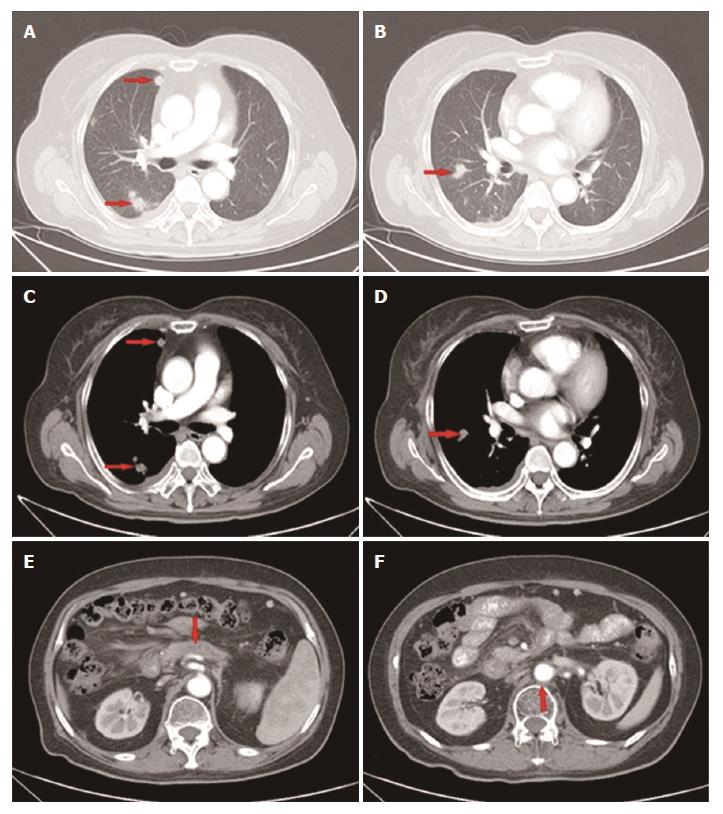
Figure 4 Computed tomography scan showed that the disease had progressed to stage IV.
Metastatic lesions in the right pleura and lungs (lung window A, B; mediastinal window C, D); The lesion in the pancreas and enlarged lymph node remained stable (E, F).
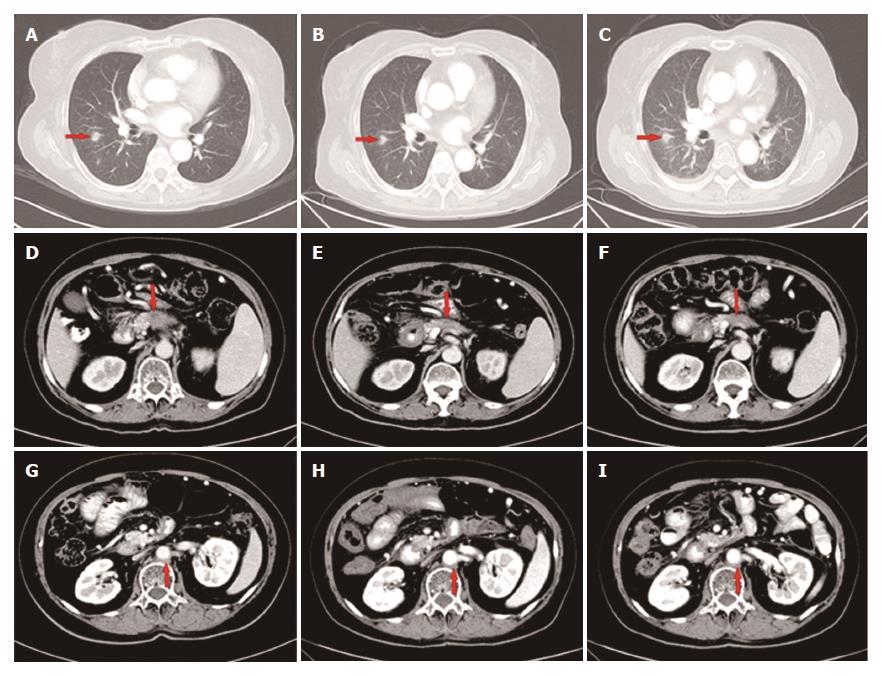
Figure 5 The primary mass in the pancreas shrunk gradually during apatinib treatment: (D) 1.
7 cm (E) 1.2 cm (F) 1.0 cm. The metastatic lesions showed no obvious change (A-C; G-I).
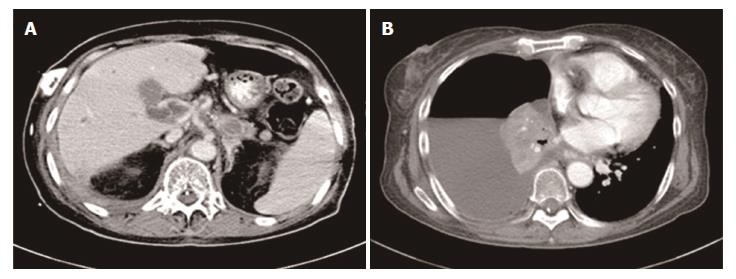
Figure 6 Disease progression.
CT scan revealed several metastatic lesions in the liver (A) and pleural effusion (B). CT: Computed tomography.
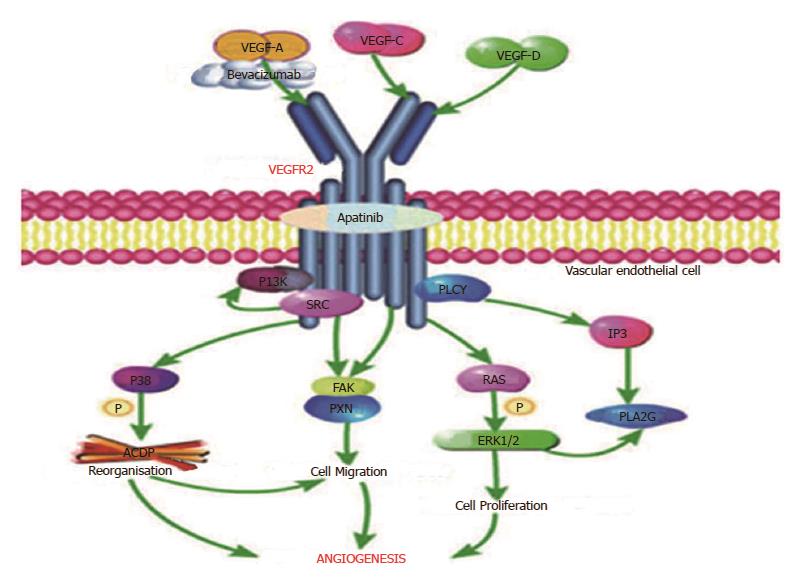
Figure 7 Pathway of VEGFR-2 (produced using the Pathway Builder Tool).
Bevacizumab targets VEGF-A to prevent its interaction with VEGFR-2; apatinib mainly targets VEGFR-2. VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor.
- Citation: Li CM, Liu ZC, Bao YT, Sun XD, Wang LL. Extraordinary response of metastatic pancreatic cancer to apatinib after failed chemotherapy: A case report and literature review. World J Gastroenterol 2017; 23(41): 7478-7488
- URL: https://www.wjgnet.com/1007-9327/full/v23/i41/7478.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i41.7478