Copyright
©The Author(s) 2017.
World J Gastroenterol. Aug 7, 2017; 23(29): 5395-5404
Published online Aug 7, 2017. doi: 10.3748/wjg.v23.i29.5395
Published online Aug 7, 2017. doi: 10.3748/wjg.v23.i29.5395
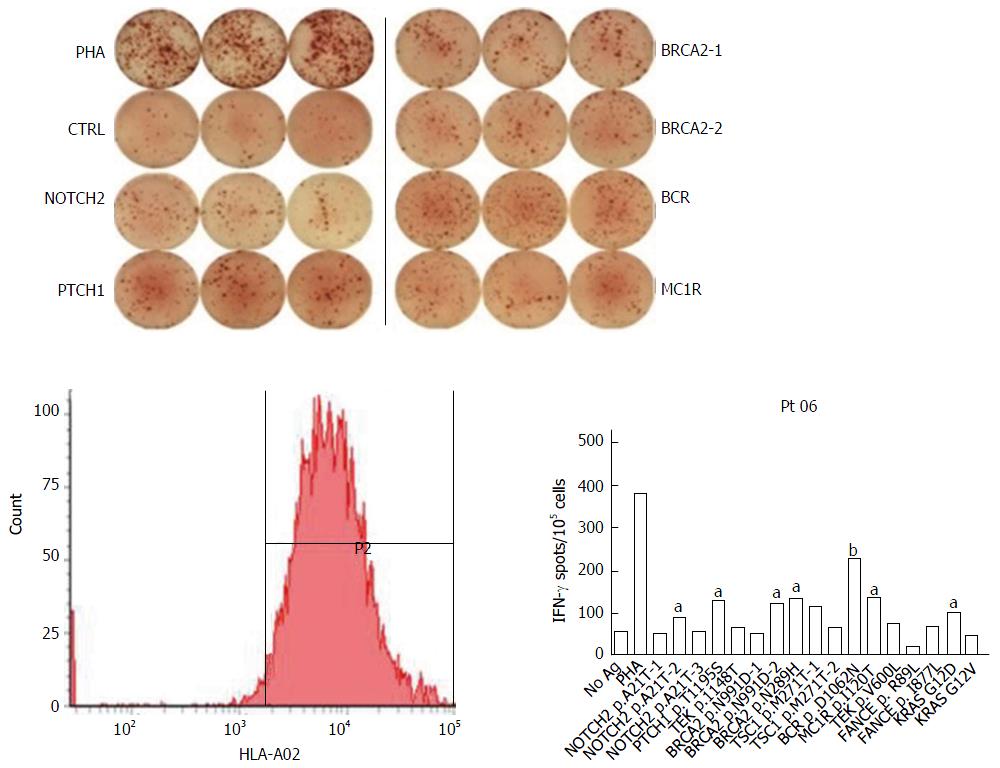
Figure 1 Selection personalized peptide from the peptide candidate library by peptide-specific IFN-γ production assay in consideration of the pre-existing host immunity.
aP < 0.05, bP < 0.01.
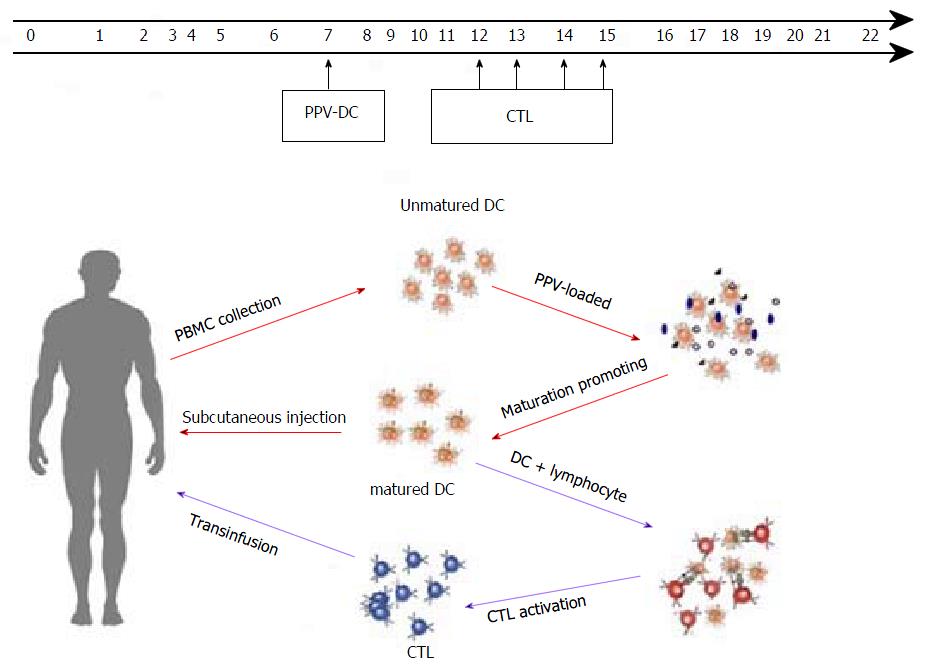
Figure 2 Time schedule of collection of peripheral blood mononuclear cells and transfusion of DC-CTL.
PPV: Personalized peptide vaccination; DC: dendritic cells; CTL: Cytotoxic lymphocytes; PBMC: Peripheral blood mononuclear cells.
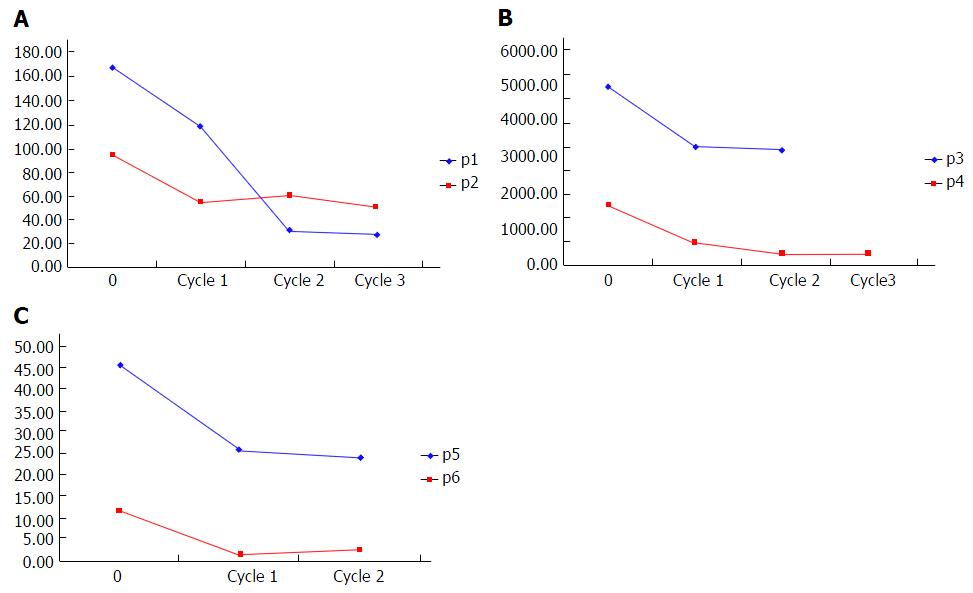
Figure 3 Changes of alpha-fetoprotein levels in patients 1-6 before and after treament.
A: AFP levels of patients 1 and 2; B: AFP levels of patients 3 and 4; C: AFP levels of patients 5 and 6. AFP: Alpha-fetoprotein.
Figure 4 Treatment outcome of patient 1.
The liver mass within and out of the radiation field were both significantly decreased in size after treatment with a combination of radiotherapy and PPV-DC-CTL. AFP had a significant decline as well.
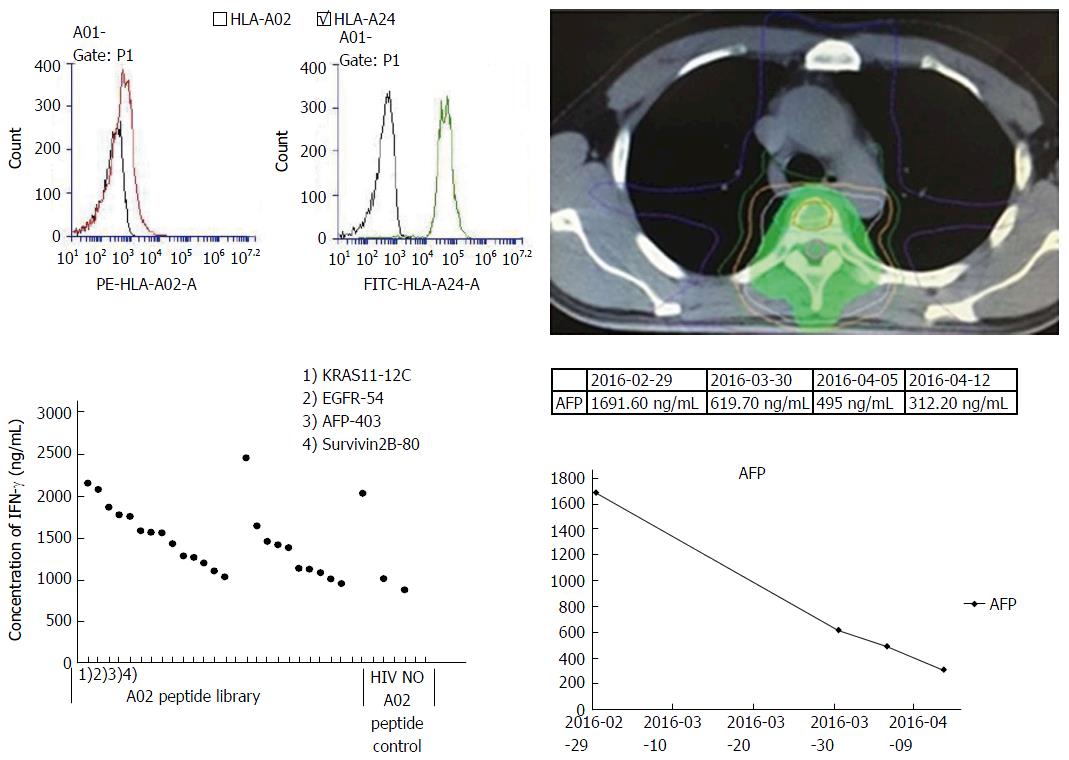
Figure 5 Treatment outcome of patient 4.
Patient 4 was a case with tumor metastasis in T4 vertebra and the lung. After treatment with a combination of radiotherapy and PPV-DC-CTL, AFP had a significant decline and chest pain was relieved.
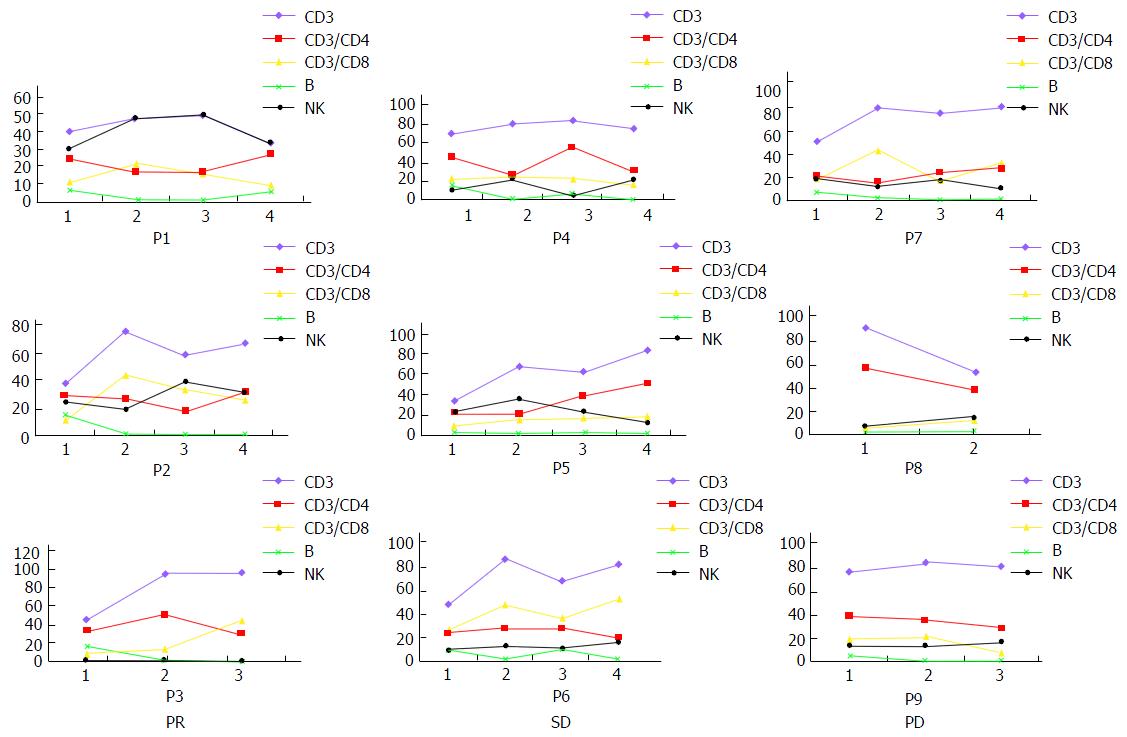
Figure 6 Cellular immune responses specific to the treatment.
CD3+, CD8+ cytotoxic T lymphocytes and NK cells were increased after CTL transfusion in most cases, suggesting the possibility of immune activation. Other lymphocyte subsets had no significant changes. NK: Natural killer; PR: Partial response; SD: Stable disease; PD: Progressive disease.
- Citation: Shen J, Wang LF, Zou ZY, Kong WW, Yan J, Meng FY, Chen FJ, Du J, Shao J, Xu QP, Ren HZ, Li RT, Wei J, Qian XP, Liu BR. Phase I clinical study of personalized peptide vaccination combined with radiotherapy for advanced hepatocellular carcinoma. World J Gastroenterol 2017; 23(29): 5395-5404
- URL: https://www.wjgnet.com/1007-9327/full/v23/i29/5395.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i29.5395