Copyright
©The Author(s) 2016.
World J Gastroenterol. Dec 28, 2016; 22(48): 10631-10642
Published online Dec 28, 2016. doi: 10.3748/wjg.v22.i48.10631
Published online Dec 28, 2016. doi: 10.3748/wjg.v22.i48.10631
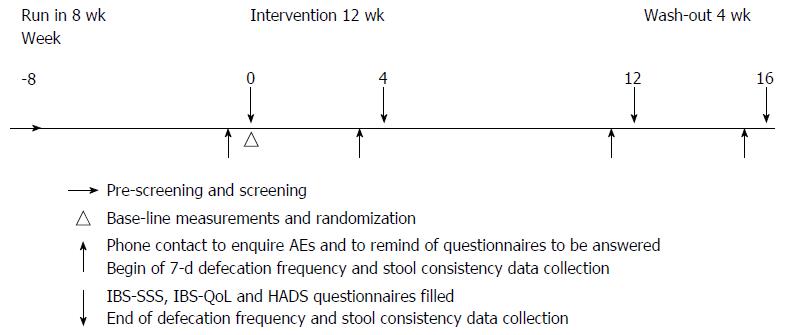
Figure 1 Study outline.
Volunteers were selected for screening visits by a prescreen for compliance over the phone. Eligible volunteers entered an 8-wk run-in period without any investigational product (IP) consumption and were thereafter randomized to receive low- or high-dose active IP or a placebo IP for 12 wk. The intervention period was followed by a 4-wk washout period without any IP consumption. Preceding each time point (weeks 0, 4, 12, and 16), volunteers recorded their stool consistency during each defecation event for 7 d. At each time point, questionnaires [Irritable Bowel Syndrome Symptom Severity Score (IBS-SSS); IBS-related Quality of Life (IBS-QoL); Hospital Anxiety and Depression Score (HADS)] were filled out. All volunteers were contacted by phone prior to each sampling time point to inquire about adverse events (AEs) and to remind of the sampling procedures.
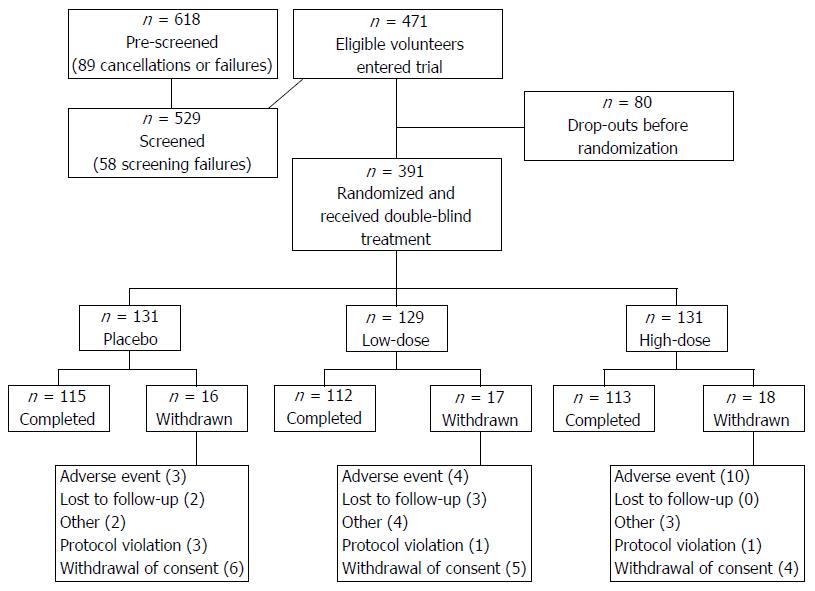
Figure 2 Disposition of volunteers.
During the 8-wk run-in period prior to randomization, 17% of eligible volunteers dropped out. After randomization, most volunteers (87%) completed the trial. Withdrawals were evenly distributed between treatment groups. Low- and high-dose treatment groups received 109 or 1010 CFU Lactobacillus acidophilus NCFM daily.
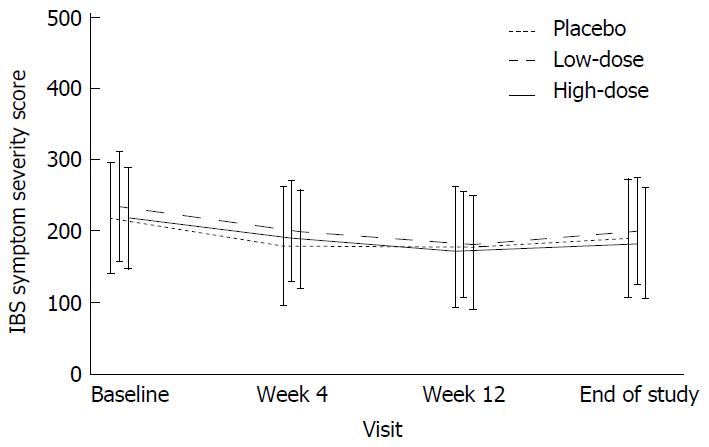
Figure 3 Irritable bowel syndrome symptom severity score over time.
IBS-SSS is a 5-item composite score inquiring about the severity of abdominal pain, bloating/distension, satisfaction with bowel habits, and IBS-related quality of life on a 10-cm VAS scale and the number of days with abdominal pain over the past 10 d[18]. All items are scored from 0 to 100, allowing for IBS-SSSs to range from 0 to 500. Francis and colleagues have validated non-IBS controls and volunteers suffering from mild, moderate, and severe IBS symptoms to range between 0-75, 75-175, 175-300, and 300-500, respectively[18]. The composite symptom score declined similarly in all treatment groups during the intervention, showing no treatment effect for the active groups (low-dose and high-dose) receiving Lactobacillus acidophilus NCFM. The severity scores are given as mean ± SD. Differences from baseline to week 12 were not significant between treatment groups. All within-group comparisons to baseline (week 4, week 12, and washout) were significant (P < 0.001). IBS-SSS: Irritable bowel syndrome symptom severity score; IBS: Irritable bowel syndrome.
- Citation: Lyra A, Hillilä M, Huttunen T, Männikkö S, Taalikka M, Tennilä J, Tarpila A, Lahtinen S, Ouwehand AC, Veijola L. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol 2016; 22(48): 10631-10642
- URL: https://www.wjgnet.com/1007-9327/full/v22/i48/10631.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i48.10631