Copyright
©The Author(s) 2016.
World J Gastroenterol. Nov 28, 2016; 22(44): 9706-9717
Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9706
Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9706
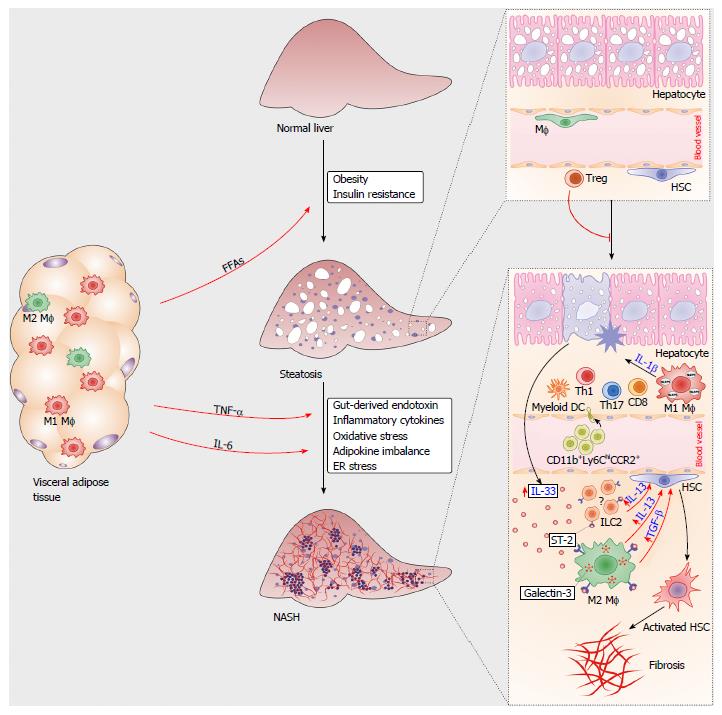
Figure 1 Immune cells in the pathogenesis of nonalcoholic steatohepatitis.
During obesity, proinflammatory M1 macrophages and Th1-type lymphocytes infiltrated in the visceral adipose tissue mediate metaflammation that triggers insulin resistance. Increased amounts of free fatty acids (FFAs) released from adipose tissue accumulate in hepatocytes, causing liver steatosis. Liver regulatory T cells suppress metabolic inflammation. Multiple signals from visceral adipose tissue and gut polarize liver resident macrophages towards M1 type, promote chemotaxis of immune cells and hepatocyte injury. Damaged hepatocytes release IL-33, which promotes release of profibrogenic IL-13 and TGF-β from IL-33R (ST2)-positive macrophages. Liver resident innate lymphoid cells type 2 (ILC2s) might also respond to IL-33 by producing IL-13. Profibrogenic cytokines activate quiescent hepatic stellate cells, which transform to myofibroblasts, the key cells involved in the development of liver fibrosis.
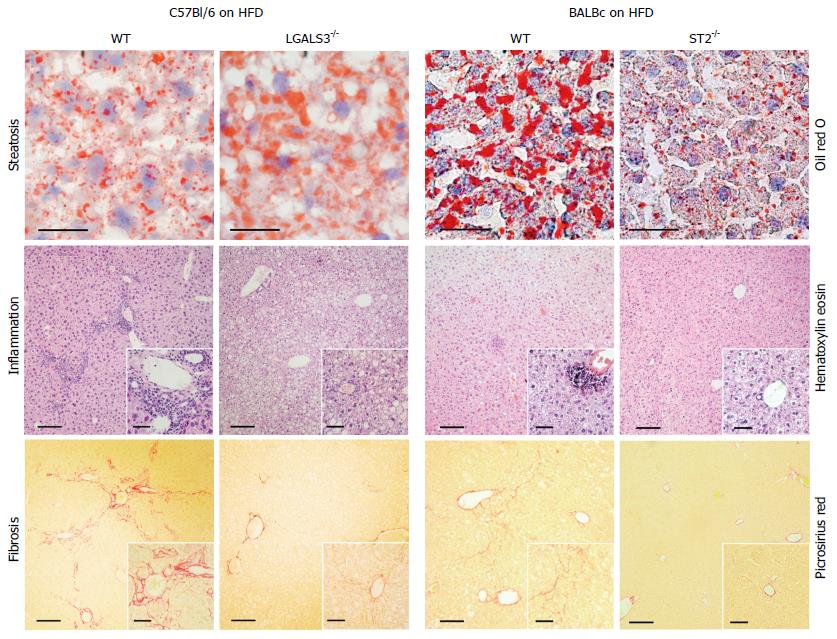
Figure 2 Galectin-3 and IL-33/ST2 axis in diet-induced steatohepatitis.
Increased liver steatosis, but attenuated inflammation and fibrosis, in Galectin-3 knockout mice fed high-fat diet compared to diet matched C57Bl/6 wild-type mice (left panel). Decreased liver steatosis, inflammation and fibrosis in ST2 knockout mice fed high-fat diet compared to diet matched BALB/c wild-type mice.
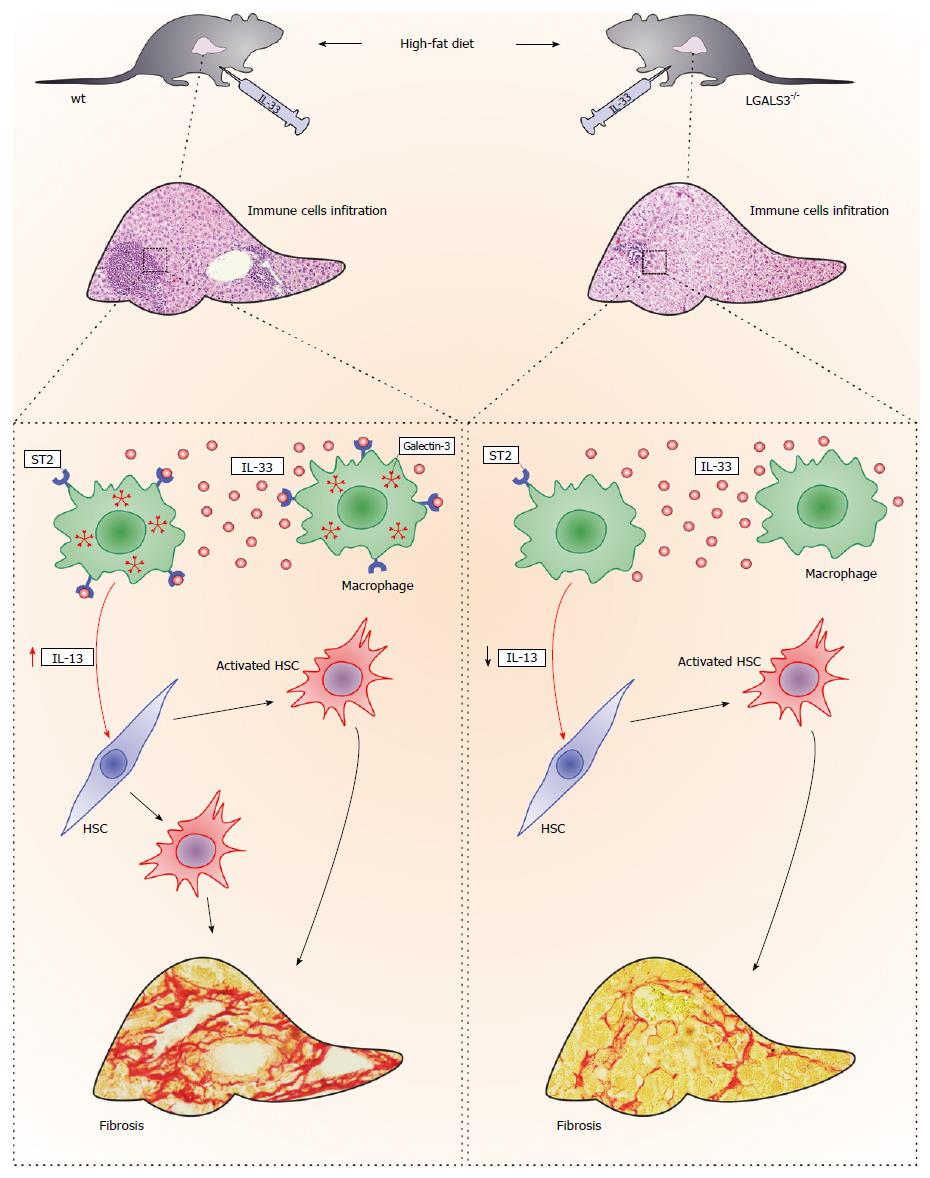
Figure 3 Galectin-3 and IL-33/ST2 axis interaction in diet-induced steatohepatitis.
Administration of IL-33 in vivo enhanced high-fat diet-induced liver fibrosis in both genotypes of mice, although to a markedly lower extent in the galectin-3 knockout mice, which was accompanied by less numerous ST2-positive myeloid cells that express IL-13. Galectin-3 plays an important regulatory role in the newly described profibrotic IL-33/ST2/IL-13 pathway in hepatic fibrosis.
- Citation: Pejnovic N, Jeftic I, Jovicic N, Arsenijevic N, Lukic ML. Galectin-3 and IL-33/ST2 axis roles and interplay in diet-induced steatohepatitis. World J Gastroenterol 2016; 22(44): 9706-9717
- URL: https://www.wjgnet.com/1007-9327/full/v22/i44/9706.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i44.9706