Copyright
©The Author(s) 2015.
World J Gastroenterol. Nov 7, 2015; 21(41): 11777-11792
Published online Nov 7, 2015. doi: 10.3748/wjg.v21.i41.11777
Published online Nov 7, 2015. doi: 10.3748/wjg.v21.i41.11777
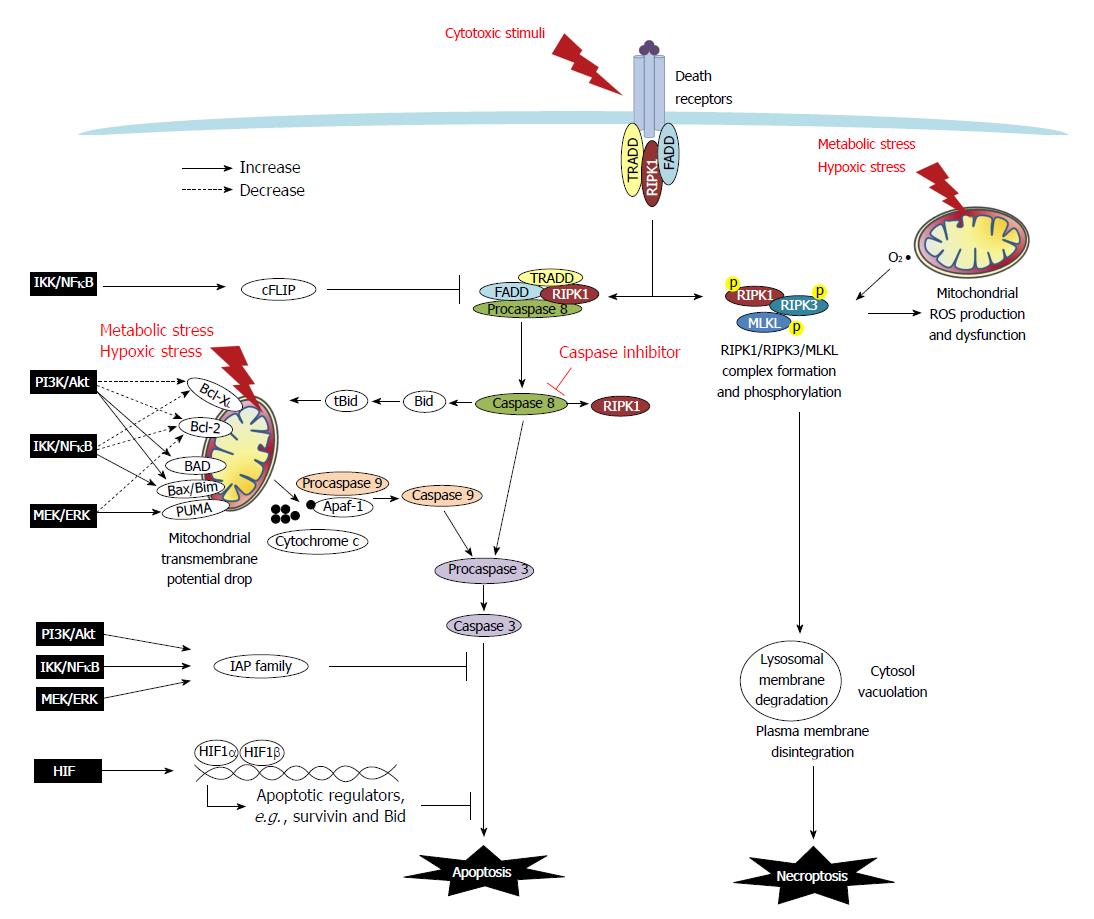
Figure 1 Death resistance signaling in cancer cells.
Programmed cell death (i.e., apoptosis and necroptosis) are either triggered extrinsically by cytotoxic stimuli through death receptors, or initiated intrinsically via mitochondria dysfunction caused by metabolic and hypoxic stress. In the extrinsic apoptotic pathway, tumor necrosis factor (TNF) or Fas binding to the receptors trigger the recruitment of adaptor molecules to form a death-inducing signaling complex which contains TNF receptor-associated death domain (TRADD), Fas-associated death domain (FADD), procaspase 8/FLICE, and receptor-interacting protein kinase 1 (RIPK1) to facilitate the activation of caspase 8. Caspase 8 then cleaves and activates caspase 3 (the final caspase in the apoptotic pathways), and it also truncates Bid and RIPK1. The intrinsic apoptotic pathway is associated with mitochondrial dysfunction. The ratio of Bcl-2 superfamily proteins, including anti-apoptotic Bcl-XL and Bcl-2, and pro-apoptotic Bad, Bid, Bax, Bim and PUMA, determines the formation of the mitochondrial permeability transition pore. The truncated form tBid cleaved by caspase-8 can migrate to the mitochondria to associate with Bax to increase membrane permeability. The drop of mitochondrial transmembrane potential leads to osmotic swelling and release of cytochrome c to complex with Apaf-1 and procaspase 9 which undergo cleavage into the active form of caspase 9. Caspase 9 and/or caspase-8 activates caspase-3, and ultimately leads to nuclear DNA fragmentation. Moreover, FLICE-like proteins (FLIP) and inhibitors to apoptosis proteins (IAPs), including cIAP, survivin and XIAP, provide a brake on the apoptotic cascade. In cancers, signaling pathways such as PI3K/Akt, MEK/ERK, IKK/IκB/NFκB and HIF regulate apoptosis by modulating Bcl-2 members and altering expression of FLIP and IAPs. In the extrinsic necroptotic pathway, stimulus of TNFα in the presence of a caspase inhibitor frees RIPK1 to form a complex with RIPK3 for auto- and trans-phosphorylation, which then recruits and phosphorylates MLKL. The RIPK1/RIPK3/MLKL complex causes mitochondrial dysfunction and executes subcellular features of necroptosis, such as lysosomal membrane degradation, cytosol vacuolation, plasma membrane disintegration, and ultimately cellular explosion. In the intrinsic necroptotic pathway, metabolic and hypoxic stress induces the mitochondrial production of reactive oxygen species (ROS) such as superoxide, which subsequently leads to RIPK1/3 activation and the final steps of necroptosis. However, signaling pathways to regulate necroptosis has not yet been reported.
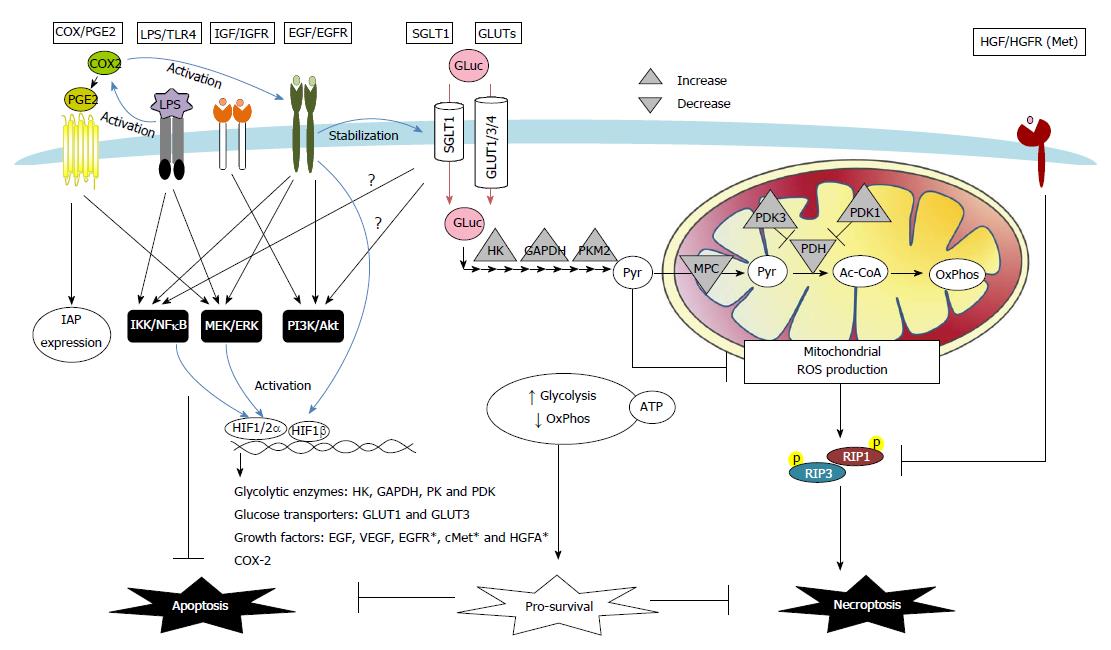
Figure 2 Proposed schema of death desistance mechanisms via modulation of receptor signaling and transporter uptake in colon cancer cells.
A number of autocrine, paracrine, or exogenous factors instigate death resistance in colon carcinoma. These pathways included cyclooxygenase (COX)-2/prostaglandin E2 (PGE2), bacterial lipopolysaccharide (LPS)/Toll-like receptor 4 (TLR4), growth factors [i.e., insulin-like growth factor (IGF), epidermal growth factor (EGF), and hepatocyte growth factor (HGF)], as well as glucose transport and metabolism. COX/PGE2 upregulates the IAP expression and activates EGF/EGFR signaling to inhibit apoptosis in colon cancer cells. TLR4 antagonizes cell apoptosis caused by its co-receptor CD14, induces anti-apoptotic MEK/ERK and IKK/IκB/NFκB signaling, and activates COX-2 pathways in colon carcinoma. Growth factors such as IGF and EGF induce anti-apoptotic PI3K/Akt, MEK/ERK, and IKK/IκB/NFκB pathways in colon cancers. Moreover, activation of HGF and its receptor Met renders colon cancer cells resistant to necroptosis via downregulation of RIPK1 protein expression. Alteration of transport and metabolism of glucose (Gluc) is another survival strategy of cancer cells. Abnormally expressed sodium-dependent glucose transporter 1 (SGLT1) and GLUT1/3/4 enhance glucose uptake in colon carcinoma. Activation of SGLT1 induces PI3K/Akt and IKK/IκB/NFκB pathways in normal intestinal epithelial cells; however, their roles in anti-death mechanisms of colon cancers remain unclear (?). Increased glycolysis and decreased mitochondria-dependent oxidative phosphorylation (OxPhos) are commonly seen in cancer cells. The metabolic shift results from upregulated expression of glycolytic enzymes for increased (Pyr) production [e.g., hexokinase (HK), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), pyruvate kinase (PK)], and also from downregulated expression of mitochondrial pyruvate carrier (MPC) and pyruvate dehydrogenase (PDH) that limits pyruvate conversion to Acetyl Co-A (Ac-CoA). The metabolic shift and predominantly glycolytic ATP generation are adaptive responses to hypoxic stress and promotes cancer cell survival. The final glycolytic product pyruvate, which is also a free radical scavenger, prevents hypoxia-induced necroptotic death in colon cancer cells via suppression of mitochondrial ROS. Hypoxia acts a stressor but also a death regulator by HIF-dependent transcription of a number of genes, including glucose metabolic enzymes [e.g., HK, GAPDH, PK, and pyruvate dehydrogenase kinase (PDK)], glucose transporters (e.g., GLUT-1 and GLUT-3), and growth factors [e.g., EGF and vascular endothelial growth factors (VEGF)]. Other HIF-targeted genes, e.g., EGFR, cMet, and HGF activator (HGFA), were reported on non-intestinal cancer cells (*), and may also contribute to the death resistance mechanisms. Lastly, EGF activates HIF signaling in normoxic conditions, leading to a positive feedback loop of adaptation fueling anti-death and pro-proliferative cancer growth.
- Citation: Huang CY, Yu LCH. Pathophysiological mechanisms of death resistance in colorectal carcinoma. World J Gastroenterol 2015; 21(41): 11777-11792
- URL: https://www.wjgnet.com/1007-9327/full/v21/i41/11777.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i41.11777