Copyright
©The Author(s) 2015.
World J Gastroenterol. Jul 21, 2015; 21(27): 8373-8381
Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8373
Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8373
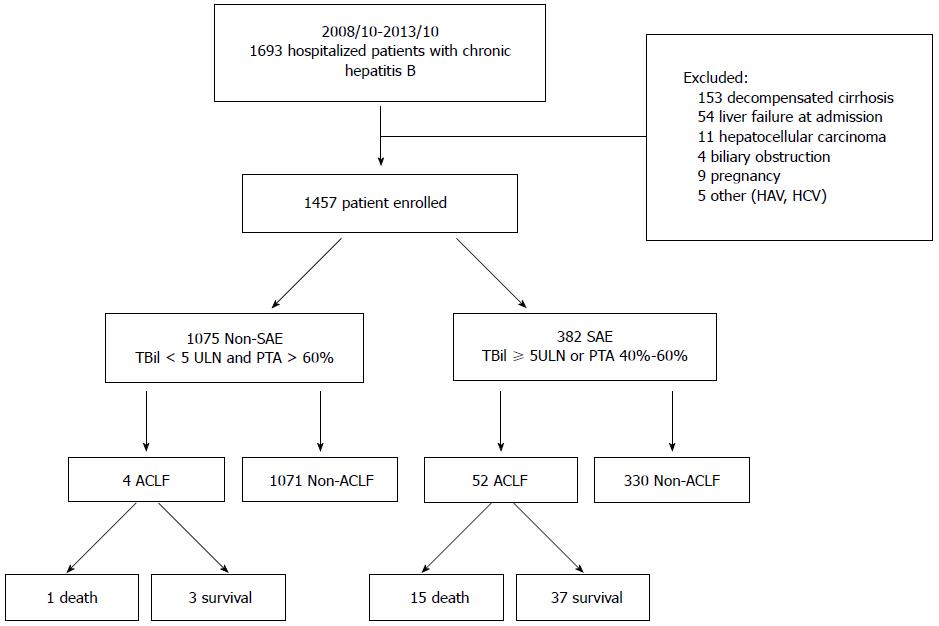
Figure 1 Outline of the screening and case selection protocol.
ACLF: Acute-on-chronic liver failure; CHB: Chronic hepatitis B; HAV: Hepatitis A virus; HCV: Hepatitis C virus; PTA: Prothrombin activity; SAE: Severe acute exacerbation; ULN: Upper limit of normal.
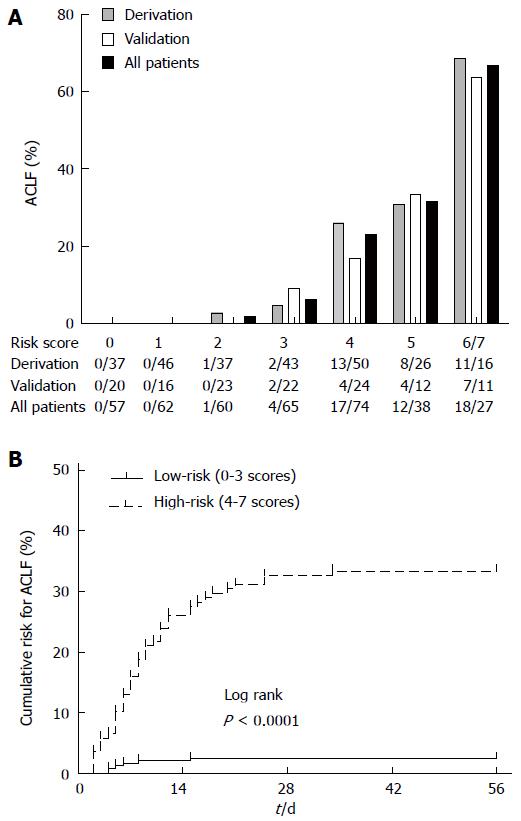
Figure 2 Risk for acute-on-chronic liver failure in patients with severe acute exacerbation with different scores.
A: The bars show the proportion of acute-on-chronic liver failure (ACLF) for each score category in the derivation, validation, and all-patient populations; B: Kaplan-Meier method compared the cumulative risk for ACLF between the low-risk (0-3 scores) and high-risk (4-7 scores) groups in the all-patient cohort.
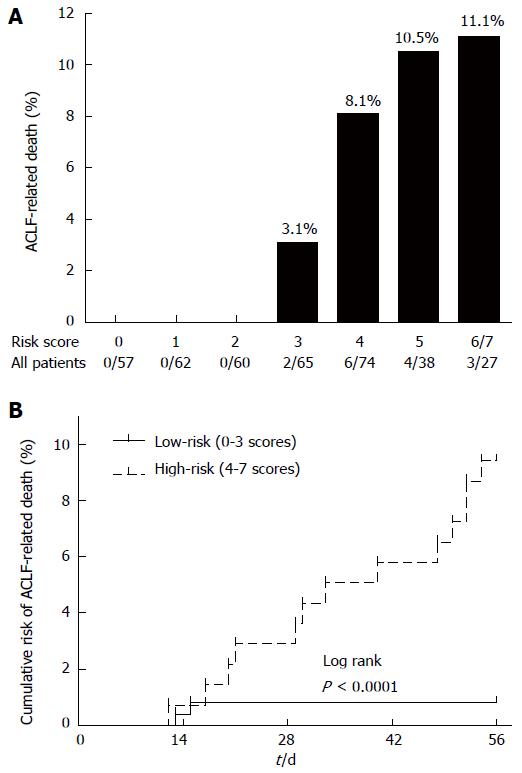
Figure 3 Risk for acute-on-chronic liver failure-related death of patients with severe acute exacerbation with different scores.
A: The bars show the proportion of acute-on-chronic liver failure (ACLF)-related death for each score category in the all-patient cohort; B: Kaplan-Meier method compared the cumulative risk of ACLF-related death between the low-risk (0-3 scores) and high-risk (4-7 scores) groups in the all-patient cohort.
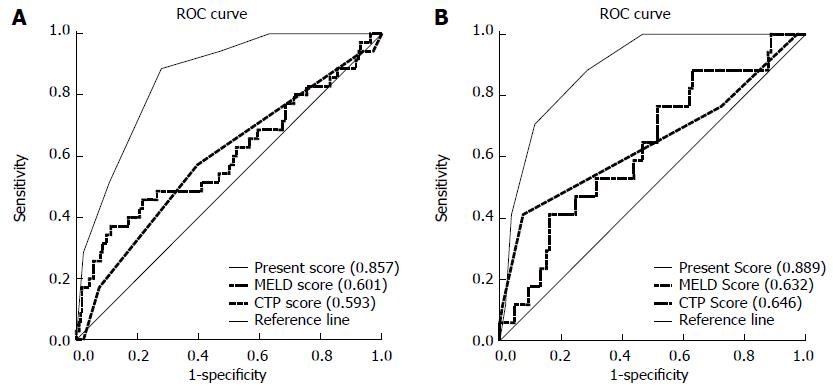
Figure 4 Area under the receiver operating characteristic curves of different score models for patients with severe acute exacerbation in the derivation and validation sets.
A: C indices for the score model in the derivation cohort; B: C indices for the score model in the validation cohort. CTP: Child-Turcotte-Pugh; MELD: Model for End-Stage Liver Disease.
- Citation: Gao FY, Liu Y, Li XS, Ye XQ, Sun L, Geng MF, Wang R, Liu HM, Zhou XB, Gu LL, Liu YM, Wan G, Wang XB. Score model for predicting acute-on-chronic liver failure risk in chronic hepatitis B. World J Gastroenterol 2015; 21(27): 8373-8381
- URL: https://www.wjgnet.com/1007-9327/full/v21/i27/8373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i27.8373