Copyright
©The Author(s) 2015.
World J Gastroenterol. Jul 21, 2015; 21(27): 8340-8351
Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8340
Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8340
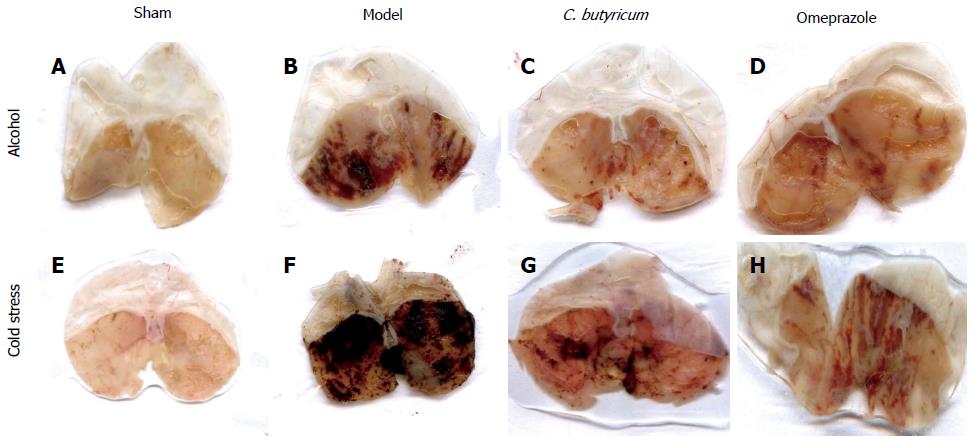
Figure 1 Macroscopic morphology of the gastric mucosa following alcohol or cold stress treatments.
The gross morphology of gastric mucosa was observed in gastric ulcer (GU) mice challenged with alcohol (A-D) or cold stress (E-H). In alcohol or cold stress-induced GU model, 40 mice were evenly divided into Sham control group (A, E), Model group (without pretreatment) (B, F), Clostridium butyricum (C. butyricum) pretreatment group (C, G) and omeprazole pretreatment group (D, H), respectively.
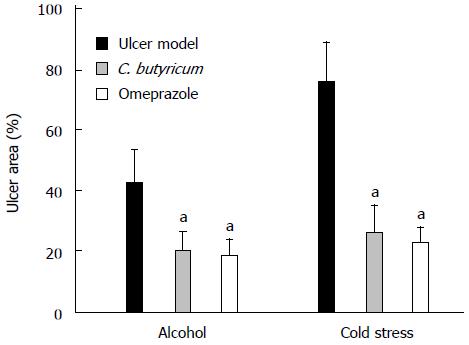
Figure 2 Effects of Clostridium butyricum on the area of gastric ulcers induced by alcohol or cold stress.
Gastric ulcer (GU) models were induced by alcohol and cold stress, and the gastric ulcer area was measured for the comparison among different groups, i.e., Model group (without pretreatment), C. butyricum pretreatment group and Omeprazole pretreatment group. The data were expressed as mean ± SD (n = 4). aP < 0.01 vs Model group.
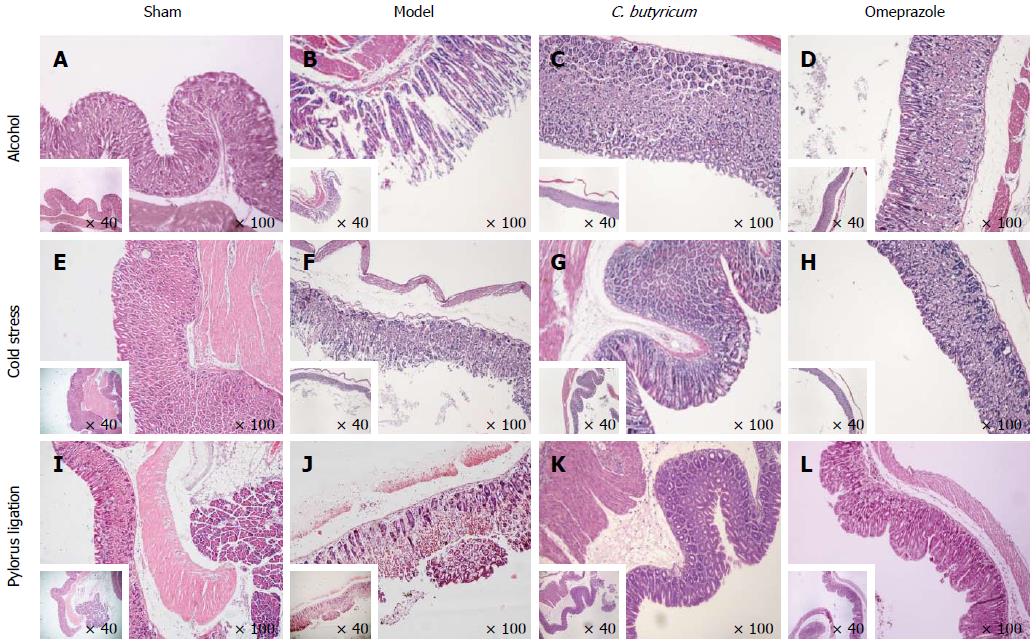
Figure 3 Histological assessment of the effect of Clostridium butyricum on acute gastric mucosal injuries in mice challenged with alcohol, cold stress or pylorus ligation.
Three different gastric ulcer (GU) models were induced by alcohol (A-D), cold stress (E-H) or pylorus ligation (I-L), and gastric tissue sections were prepared for the observation under microscope at × 40 and × 100 magnifications. For each gastric ulcer (GU) model, mice were evenly divided into a Sham control group (A, E, I), Model group (without pretreatment) (B, F, J), Clostridium butyricum (C. butyricum) pretreatment group (C, G, K) and Omeprazole pretreatment group (D, H, L). The results represent three independent experiments (n = 4).
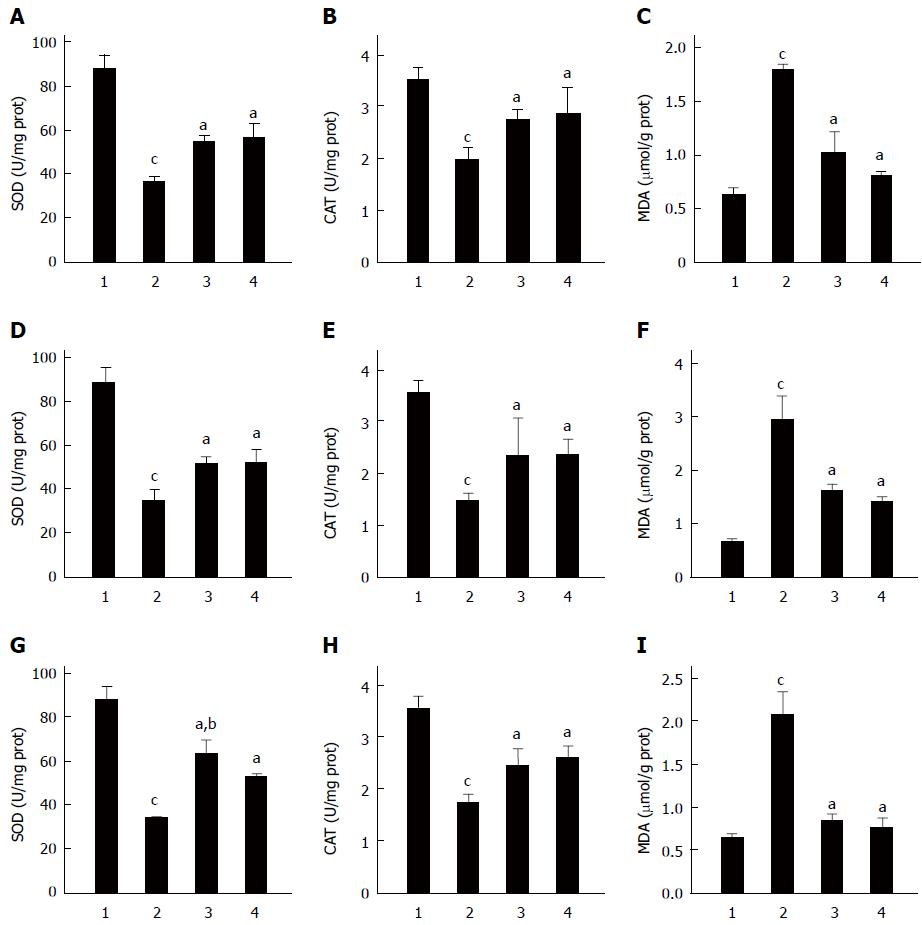
Figure 4 Effect of Clostridium butyricum on the activities of superoxide dismutase, catalase and the content of malondialdehyde in the gastric mucosa of mice with gastric ulcers induced by alcohol, cold stress or pylorus ligation.
Gastric ulcer models were induced by alcohol (A-C), cold stress (D-F) or pylorus ligation (G-I), respectively. The activities of superoxide dismutase (SOD) (A, D, G) and catalase (CAT) (B, E, H), and the content of malondialdehyde (MDA) (C, F, I) in the gastric tissues of different gastric ulcers (GU) mice were detected. For each GU model, there were four different groups: Sham control group (1), Model group (without pretreatment) (2), C. butyricum pretreatment group (3) and Omeprazole pretreatment group (4). The data were expressed as mean ± SD (n = 6). aP < 0.01 vs Model group; bP < 0.01 vs Omeprazole group; cP < 0.01 vs Sham control group.
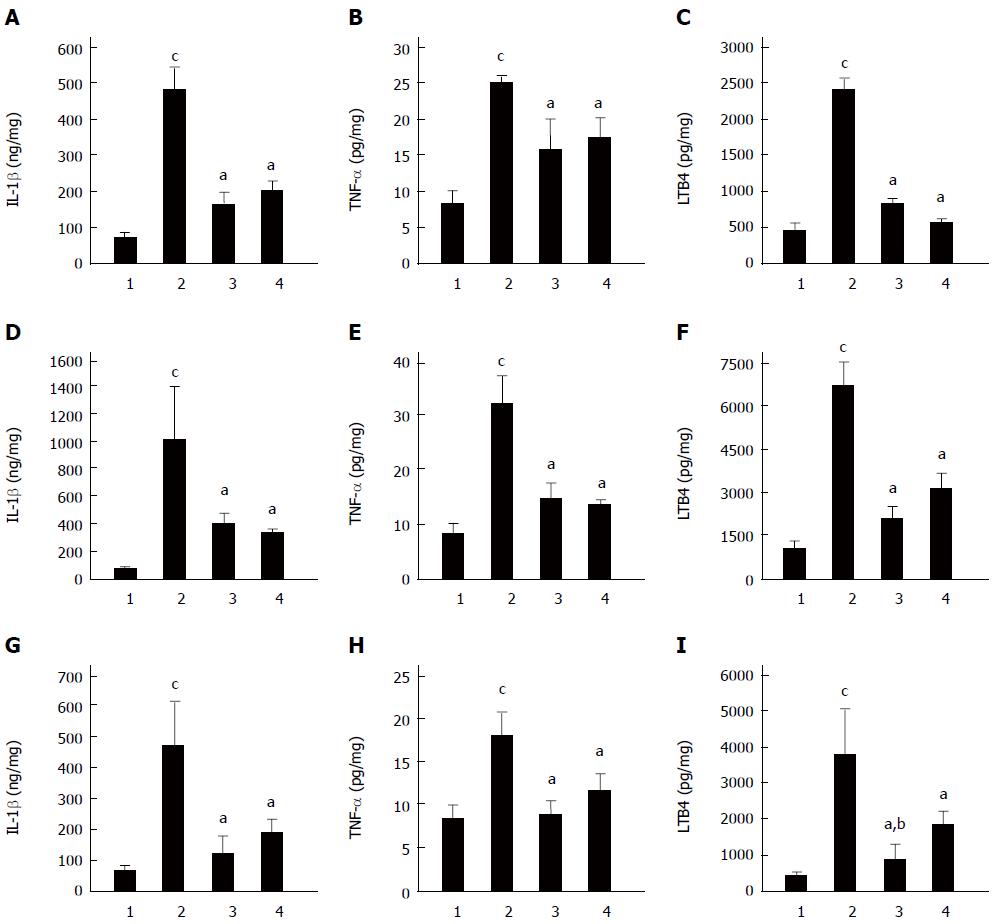
Figure 5 Effect of Clostridium butyricum on the levels of IL-1β, TNF-α and LTB4 in the gastric mucosa of mice with gastric ulcers induced by alcohol, cold stress or pylorus ligation.
Gastric ulcer models were induced by alcohol (A-C), cold stress (D-F) or pylorus ligation (G-I), respectively. The levels of IL-1β (A, D, G), TNF-α (B, E, H) and LTB4 (C, F, I) in gastric tissue of different GU mice were quantified. For each GU model, there were four different groups: Sham control group (1), Model group (without pretreatment) (2), Clostridium butyricum (C. butyricum) pretreatment group (3) and Omeprazole pretreatment group (4). The data were expressed as mean ± SD (n = 6). aP < 0.01 vs Model group; bP < 0.01 vs Omeprazole group; cP < 0.01 vs Sham control group.
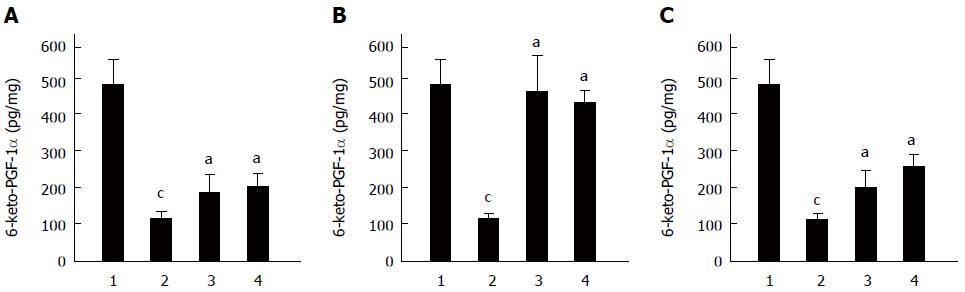
Figure 6 Effect of Clostridium butyricum on 6-keto-PGF-1α in the gastric mucosa of mice with gastric ulcers induced by alcohol, cold stress or pylorus ligation.
Gastric ulcer models were induced by alcohol (A), cold stress (B) or pylorus ligation (C), respectively. The content of 6-keto-PGF-1α in gastric tissue of different gastric ulcers (GU) mice was measured. For each GU model, there were four different groups: Sham control group (1), Model group (without pretreatment) (2), Clostridium butyricum (C. butyricum) pretreatment group (3) and Omeprazole pretreatment group (4). The data were expressed as mean ± SD (n = 6). aP < 0.01 vs Model (without pretreatment) group; cP < 0.01 vs Sham control group.
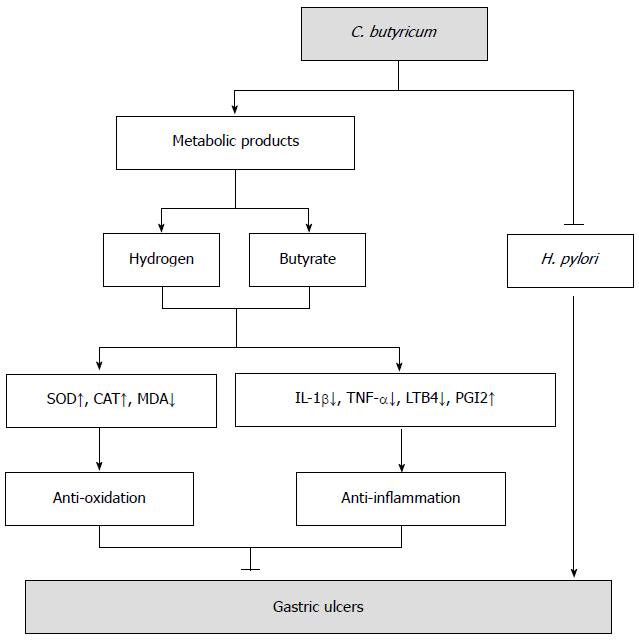
Figure 7 The hypothetical model of Clostridium butyricum protective effect on gastric ulcers induced by stressful stimuli.
In addition to competitive inhibition of Helicobacter pylori, as reported by previous studies[17], Clostridium butyricum (C. butyricum) protects gastric mucosa from lesions through at least two different mechanisms: anti-oxidation and anti-inflammation. The metabolites of C. butyricum, such as butyrate and hydrogen[52], enhance the activity of antioxidases [superoxide dismutase (SOD) and catalase (CAT)], reduce the production of proinflammatory factors [interleukin (IL)-1β, tumor necrosis factor (TNF)-α, leukotriene B4 (LTB4)] and promote the production of anti-inflammatory prostaglandins (PGI2), thus exerting protective effect on gastric ulcers by resolving peroxidation and alleviating exaggerated inflammation induced by various stresses.
-
Citation: Wang FY, Liu JM, Luo HH, Liu AH, Jiang Y. Potential protective effects of
Clostridium butyricum on experimental gastric ulcers in mice. World J Gastroenterol 2015; 21(27): 8340-8351 - URL: https://www.wjgnet.com/1007-9327/full/v21/i27/8340.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i27.8340