Copyright
©The Author(s) 2015.
World J Gastroenterol. May 14, 2015; 21(18): 5677-5684
Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5677
Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5677
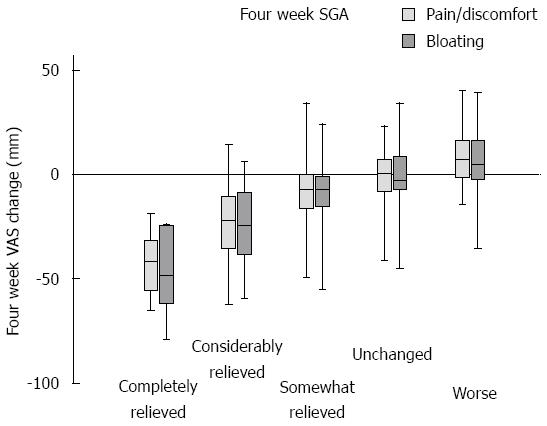
Figure 1 Scale and precision of the subjective global assessment measure.
At 4 wk, the change in VAS registration (compared to pre-registration values) was calculated. Box and whiskers plot of VAS change in the different subcategories of SGA at 4 wk. It is noted that the scale is not entirely linear, with best discrimination in the left part of the plot, while the right part shows smaller VAS differences between groups. SGA: Subjective global assessment; VAS: Visual analog scale.
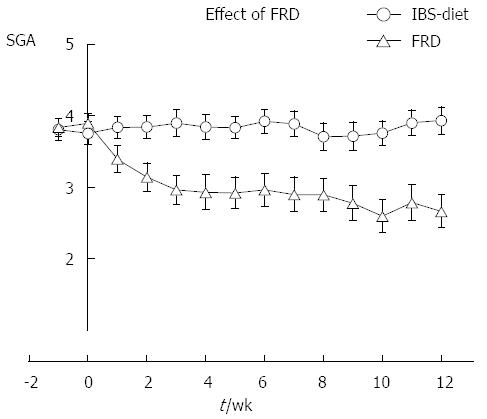
Figure 2 Subjective global assessment of irritable bowel syndrome-related symptoms during the whole study.
Mean registration (95%CI) for study groups. The control group showed stable mean value during the 2 + 12 wk registration. The mean effect of FRD was marked, showing stable improvement of symptom rating during the whole study. The SGA ratings were: 1: Completely relieved; 2: Considerably relived; 3: Somewhat relieved; 4: Unchanged; 5: Worse. FRD: Fructose-reduced diet; SGA: Subjective global assessment; IBS: Irritable bowel syndrome.
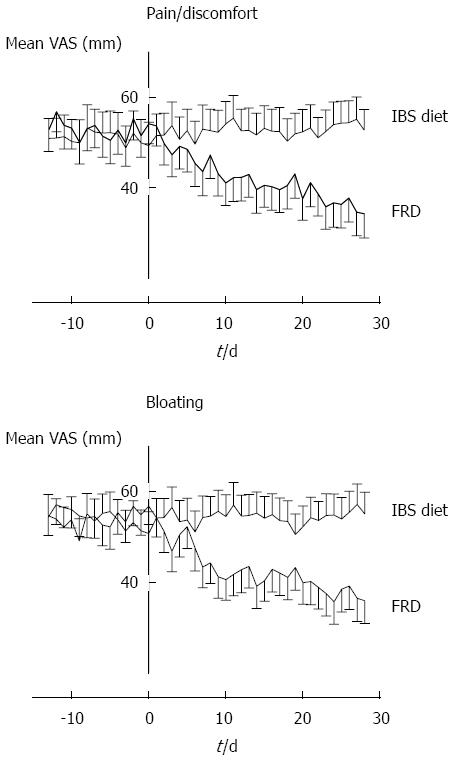
Figure 3 Visual analog scale registrations of irritable bowel syndrome-related symptoms during the first 2 + 4 wk.
Mean registration (95%CI) for the study groups. FRD: Fructose-reduced diet; VAS: Visual analog scale; IBS: Irritable bowel syndrome.
- Citation: Berg LK, Fagerli E, Myhre AO, Florholmen J, Goll R. Self-reported dietary fructose intolerance in irritable bowel syndrome: Proposed diagnostic criteria. World J Gastroenterol 2015; 21(18): 5677-5684
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5677.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5677