Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Nov 21, 2014; 20(43): 16037-16052
Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16037
Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16037
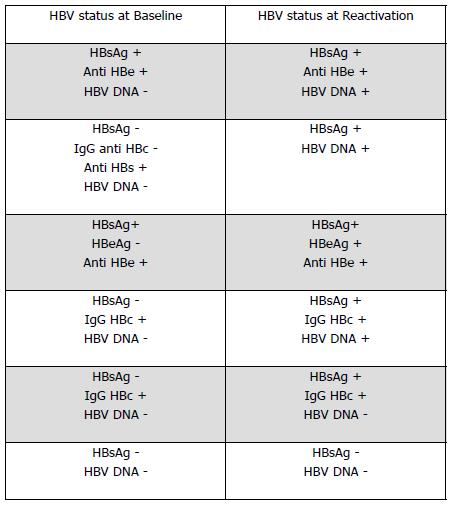
Figure 1 Serological and virological profiles in reactivation of chronic hepatitis B.
HBV: Hepatitis B virus; HBeAg: Hepatitis B e antigen.
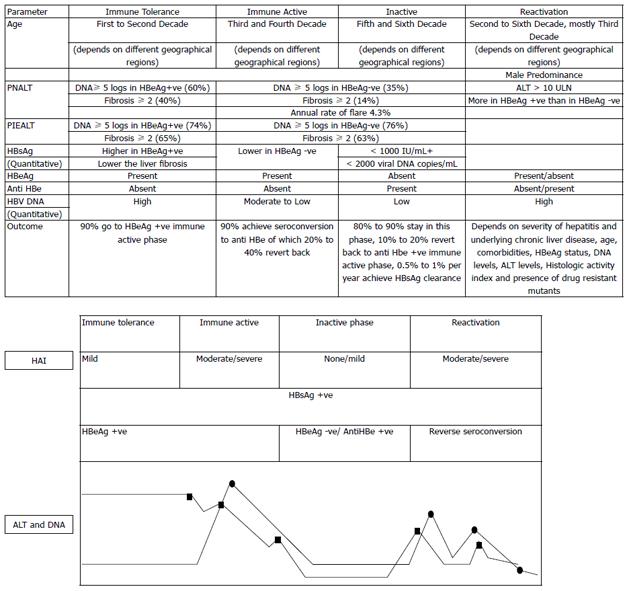
Figure 2 Natural history of chronic hepatitis B virus infection and phases of reactivation.
The variations in alanine aminotransferase (ALT) and DNA levels are shown in relation to histological activity index (HAI) (also shown in the inset). ALT (circles) and HBV DNA (squares). PIEALT: persistently or intermittently elevated ALT [defined as ≥ 3 ALT values in the year prior to baseline liver biopsy, and all values were > 40 IU/L and had remained so until the start of treatment or last follow-up if not treated (persistently elevated) or if they had ≥ 3 ALT values and ≥ 1 > 40 IU/L in the year prior to baseline biopsy or anytime until the start of treatment or last follow-up, if not treated (intermittently elevated)]. PNALT: Persistently normal ALT (defined as ≥ 3 ALT values in the year prior to baseline liver biopsy, and all values were < 40 IU/L and had remained so until the start of treatment or last follow-up if not treated). HBeAg: Hepatitis B e antigen.
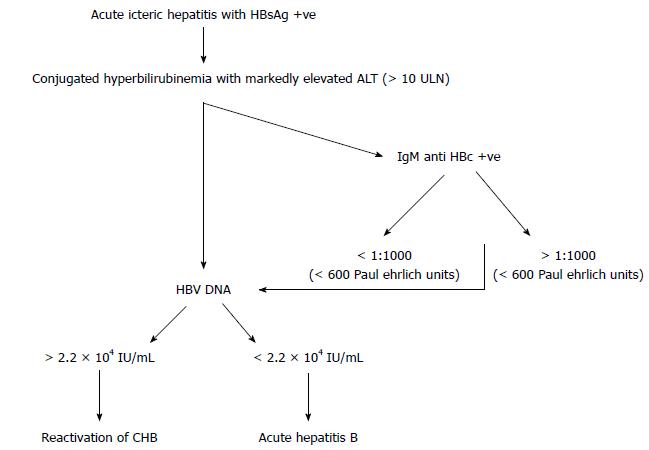
Figure 3 Algorithm for diagnosis of acute viral hepatitis B vs hepatitis B reactivation.
HBeAg: Hepatitis B e antigen; ALT: Alanine aminotransferase.
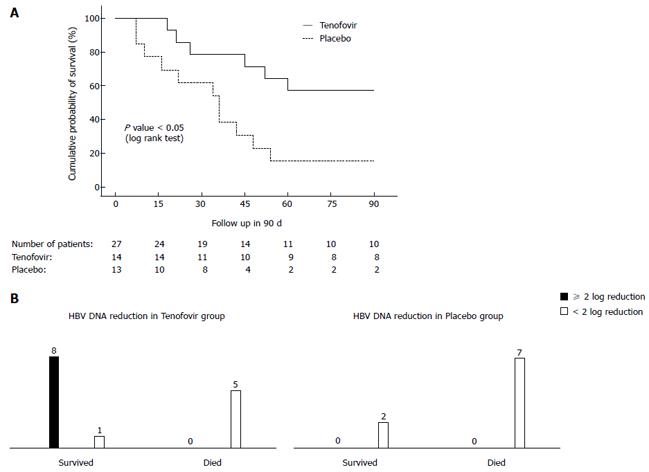
Figure 4 Cumulative survival of two groups of acute on chronic liver failure-hepatitis B virus patients treated with tenofovir vs placebo is shown in the Kaplan-Meier graph below.
A: Cumulative survival of acute on chronic liver failure-hepatitis B virus (ACLF-HBV) group of patients who were randomized to receive tenofovir vs placebo. Of the 90 patients with ACLF of different etiologies, 27 (26%) were due to reactivation of chronic hepatitis B. The median baseline HBV DNA level was 9 × 105 IU/mL. Fourteen patients received tenofovir and 13 placebo. At 3 mo the probability of survival was higher in the tenofovir than the placebo group [8/14 (57%) vs 2/13 (15%), respectively; P < 0.03]. The cause of death in the 15 patients was progressive liver failure leading to multiorgan failure. In the surviving patients, there was a significant improvement in the Child-Turcotte Pugh (CTP) and model for end-stage liver disease (MELD) scores and significant decline in the HBV DNA levels in the tenofovir group, whereas these parameters did not change significantly in the placebo group. B: A 2-log reduction in HBV DNA level at 2 wk predicted survival benefit. Garg et al[61] 2011 (Hepatology). HBV: Hepatitis B virus.
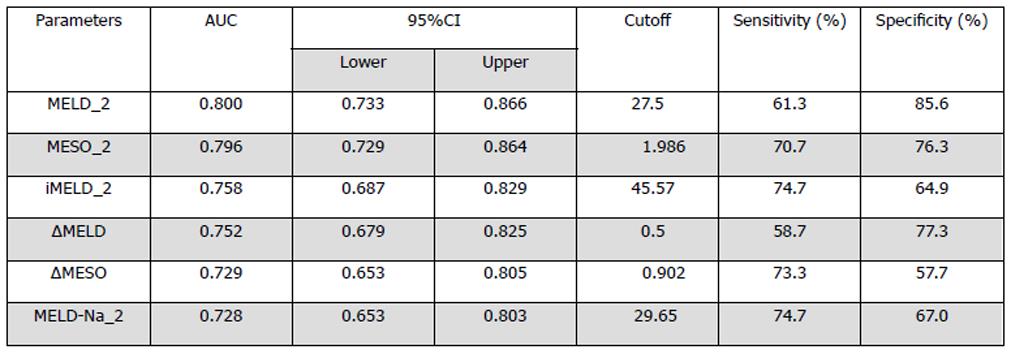
Figure 5 Sensitivity, specificity, and 95%CI for the four model for end-stage liver disease-based prognostic models used to predict mortality with the best predictive cut offs at 3 mo[67].
Figure 6 Algorithm for management of acute on chronic liver failure secondary to reactivation of chronic hepatitis B.
HBV: Hepatitis B virus.
- Citation: Philips CA, Sarin SK. Potent antiviral therapy improves survival in acute on chronic liver failure due to hepatitis B virus reactivation. World J Gastroenterol 2014; 20(43): 16037-16052
- URL: https://www.wjgnet.com/1007-9327/full/v20/i43/16037.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.16037