Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Nov 14, 2014; 20(42): 15931-15936
Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15931
Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15931
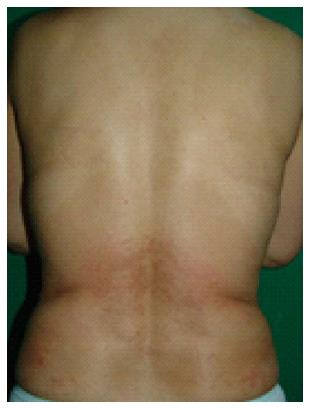
Figure 1 Cutaneous eruption associated with entecavir that manifested after one week of therapy in a non-naïve patient with chronic hepatitis B.
Coalescent erythema, intermingled with papules, was observed on the lower back.
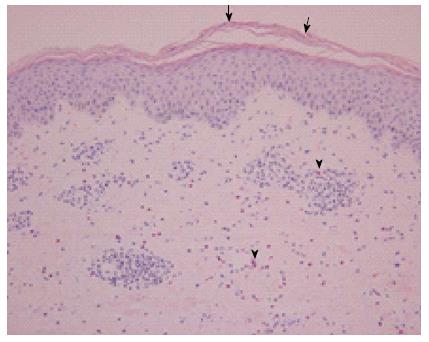
Figure 2 Hematoxylin and eosin stained erupted skin biopsy sample.
The epidermis is acanthotic with focal parakeratosis (arrows). A perivascular lymphocytic infiltrate admixed with interstitial eosinophils is present in the papillary and reticular dermis (arrowheads). Magnification: 200 ×.
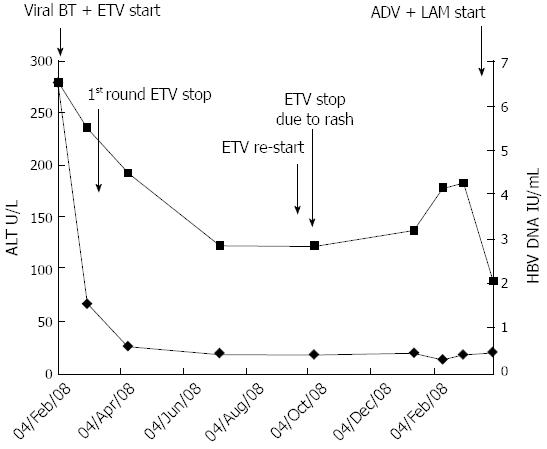
Figure 3 Alanine aminotransferase and hepatitis B virus DNA levels following the course of various antiviral treatments.
Entecavir (1 mg) treatment could not be continued past the 15th day due to an adverse drug reaction (skin rash). ALT: Alanine aminotransferas; HBV: Hepatitis B virus; ADV: Adverse drug reaction; BT: Breakthrough; ETV: Entecavir; LAM: Lamivudine.
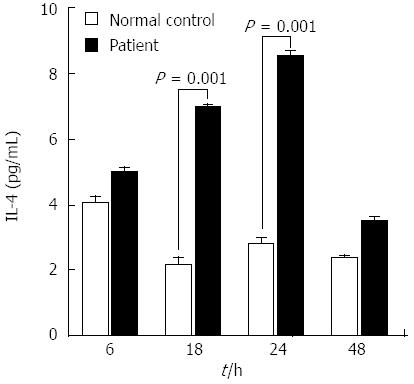
Figure 4 Cytokine release profile of peripheral blood mononuclear cells isolated from the patient during the cutaneous eruption associated with entecavir treatment.
The level of secreted interleukin (IL)-4 was significantly elevated at 18 and 24 h after entecavir treatment. IL-4 is the representative cytokine of type 4b hypersensitivity.
- Citation: Kim JT, Jeong HW, Choi KH, Yoon TY, Sung N, Choi YK, Kim EH, Chae HB. Delayed hypersensitivity reaction resulting in maculopapular-type eruption due to entecavir in the treatment of chronic hepatitis B. World J Gastroenterol 2014; 20(42): 15931-15936
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15931.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15931