Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Jan 28, 2014; 20(4): 923-942
Published online Jan 28, 2014. doi: 10.3748/wjg.v20.i4.923
Published online Jan 28, 2014. doi: 10.3748/wjg.v20.i4.923
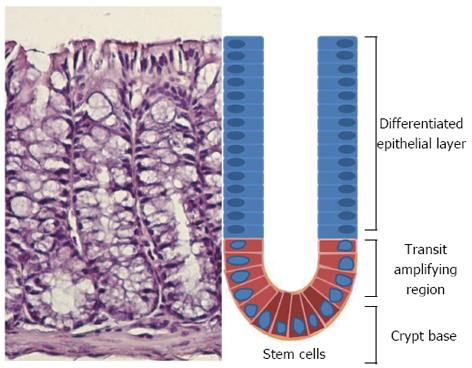
Figure 1 Schematic representation of an individual colon crypt showing the position of different cell types.
Stem cells lie at the bottom of the crypt and through an asymmetric division are responsible for generating all epithelial cell types along the crypt-villus axis.
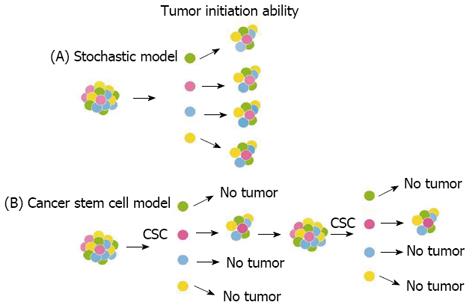
Figure 2 Models of tumor development.
(A) Stochastic model: every cancer cell isolated from the bulk tumor is tumorigenic and thus has the ability to proliferate extensively and initiate tumor growth. (B) Cancer stem cell (CSC) model: only a rare subpopulation of undifferentiated cells has the unique biological properties necessary for tumor initiation, maintenance, and spreading.
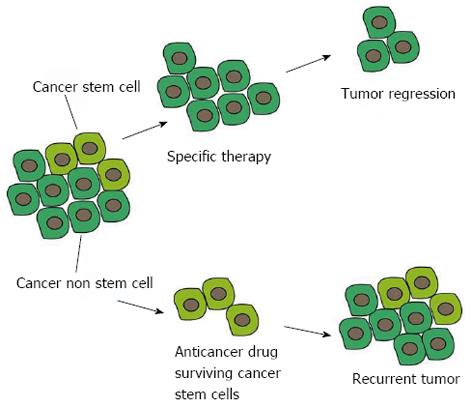
Figure 3 Advantages of a cancer stem cell-specific therapy compared to conventional anticancer therapies.
The current anticancer drugs wipe out most of the bulk population but the surviving cancer stem cells (CSCs) can repopulate the tumor. Specific targeting of CSCs is essential for regression and complete eradication of the tumor.
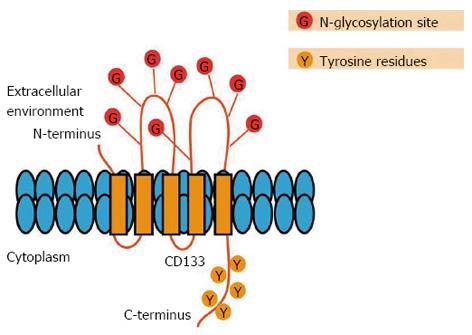
Figure 4 Schematic representation of the CD133 molecule.
CD133 consists of an extracellular N-terminal domain, a cytoplasmic C-terminus containing five tyrosine residues, two small cysteine-rich cytoplasmic loops and two large extracellular loops, each containing four consensus sequences for N-linked glycosylation.
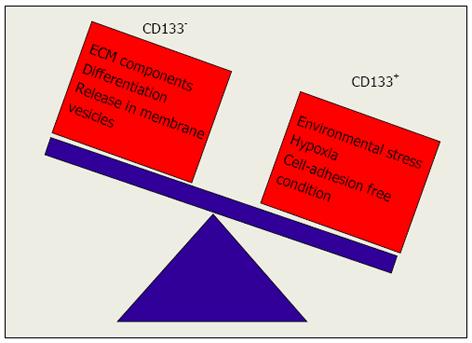
Figure 5 Signals regulating CD133 expression levels.
The exposure to environmental stress, hypoxia and cell-adhesion-free condition promotes switching of CD133- to CD133+ cells while exposure to ECM components promotes switching of CD133+ to CD133- cells.
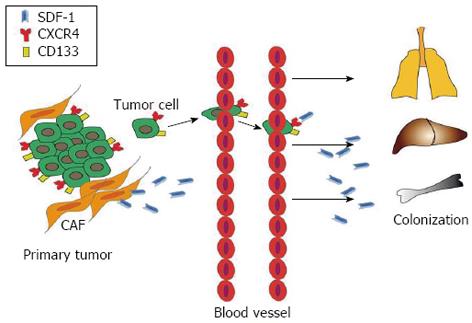
Figure 6 Possible role of the interaction between CXCR4/CD133 cancer cells and SDF-1 ligands.
The SDF-1 ligand secreted by carcinoma-associated fibroblasts (CAF) in tumor microenvironment interacts with CXCR4/CD133 expressing cancer cells and could drive primary tumor cells towards metastatic sites.
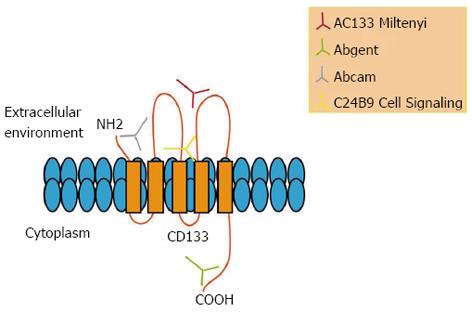
Figure 7 Epitopes recognized by different antibodies on CD133 molecule.
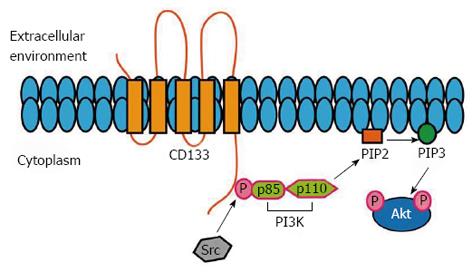
Figure 8 Potential molecular pathways associated with CD133.
The phosphorylation of the tyrosine 828 is involved in the binding to p85 (PI3K regulatory subunit) and in the subsequent activation of PI3K/Akt pathway, which, finally, promotes the self-renewal and tumor formation of CSCs. CSCs: Cancer stem cells.
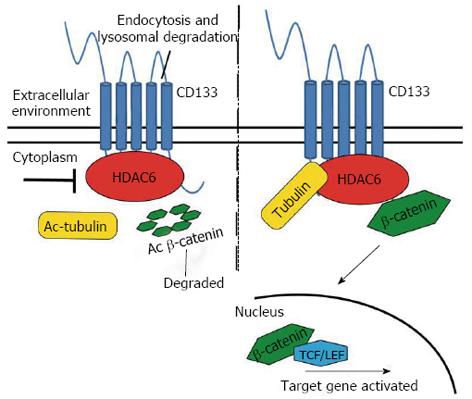
Figure 9 Schematic representation of HDAC6-mediated regulation of CD133 expression.
HDAC6 physically binds to CD133 and stabilizes the β-catenin that in the nucleus promotes the activation of its target genes. Ac: Acetylated.
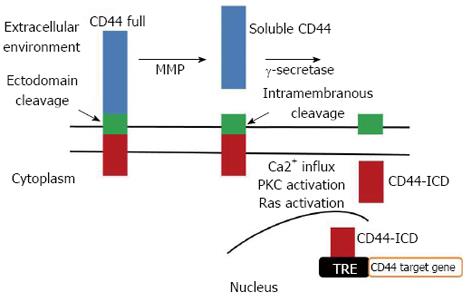
Figure 10 Schematic representation of CD44 sequential proteolytic processing.
CD44 undergoes sequential proteolytic cleavages in the ectodomain and intramembranous domain. Cleavage of CD44 ectodomain generates soluble CD44 that regulates cell attachment and migration and induces the intramembranous domain cleavage, releasing the intracellular domain of CD44 (CD44-ICD). CD44-ICD translocates to the nucleus, where it activates gene transcription, including CD44 itself, via binding to TPA-responsive elements (TRE). MMP: matrix metalloproteinase.
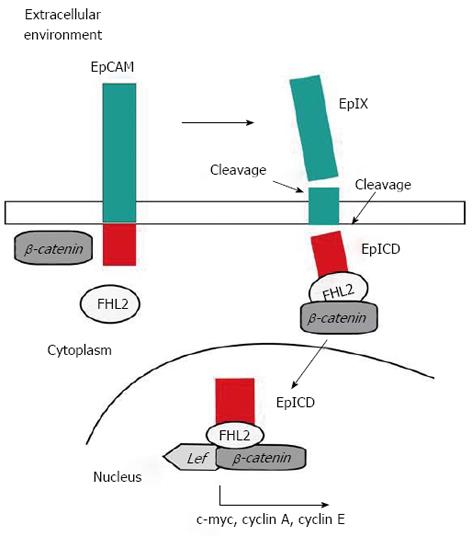
Figure 11 Schematic representation of epithelial cell adhesion molecule activation.
Cleavage of full length epithelial cell adhesion molecule (EpCAM) generates EpEx (extracellular domain of EPCAM) and EpICD (EpCAM Intracellular Domain) fragments. EpICD binds the scaffold protein FHL2 and joins to the transcriptional regulators β-catenin and, within the nucleus, interacts with LEF, binds to DNA and induces gene activation.
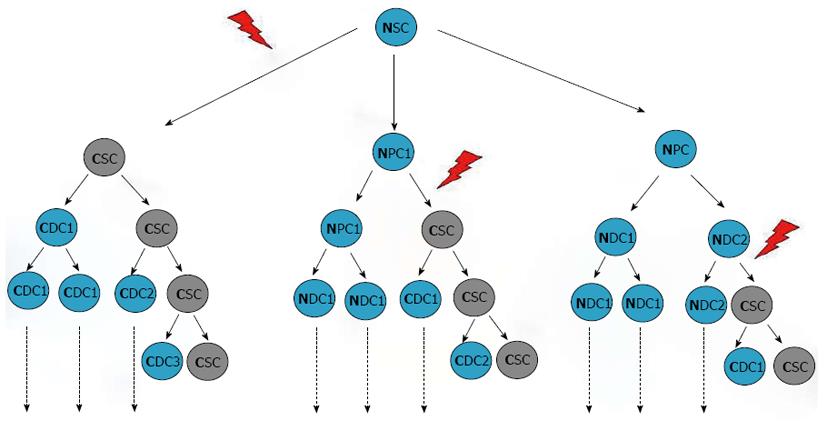
Figure 12 Origin of cancer stem cells and tumor heterogeneity.
It has been hypothesized that CSCs may derive from transformation of quiescent, normal stem cells (left) or could result from the de-differentiation of more mature cells which might re-acquire the capability of self-renewal. Other mutations might occur following transformation in both cases. SC: Stem cell; PC: Progenitor cell; DC: Differentiated cell; C: Cancer; N: Normal; CSCs: Cancer stem cells.
- Citation: Fanali C, Lucchetti D, Farina M, Corbi M, Cufino V, Cittadini A, Sgambato A. Cancer stem cells in colorectal cancer from pathogenesis to therapy: Controversies and perspectives. World J Gastroenterol 2014; 20(4): 923-942
- URL: https://www.wjgnet.com/1007-9327/full/v20/i4/923.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i4.923