Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Sep 28, 2014; 20(36): 13119-13126
Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.13119
Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.13119
Figure 1 Hepatocyte isolation with a modified four-step retrograde perfusion technique.
Perfusion was conducted by inserting a suitable pipette into vessels exposed on a cut surface of the sample (A). After sufficient digestion, the liver capsule was mechanically disrupted (B). The emerging cell suspension was filtrated through a 250 μm nylon mesh (C) and centrifuged (50 g, 2 min, 4 °C) (D).
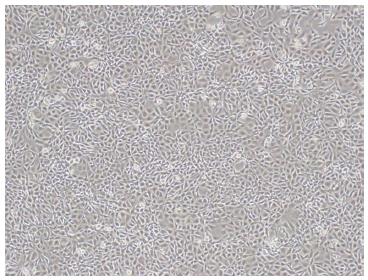
Figure 2 Morphology of SSR#69 cultured in DMEM medium supplemented with 10% newborn calf serum (× 100).
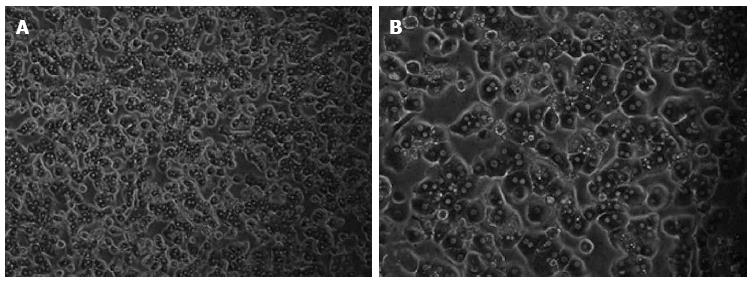
Figure 3 Morphology of freshly isolated primary human hepatocytes.
The primary human hepatocytes attached to the plates showed the typical morphological appearance with the polygonal shape, granular cytoplasm and one or more nuclei.
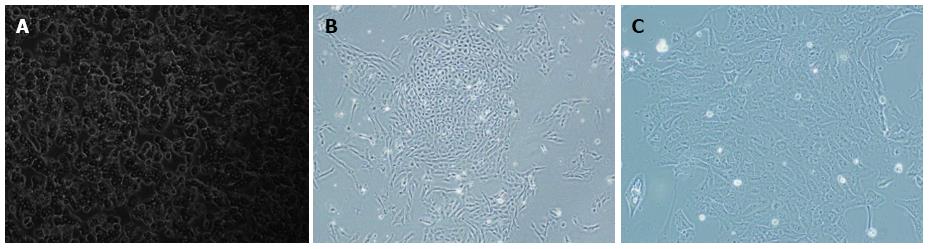
Figure 4 Morphology of primary and immortalized cells.
Non-immortalized primary hepatocytes grow slowly with low plating efficiency and a short life span (A). Immortalized human hepatocytes grow rapidly as islands and had an extended life span (B, C). Magnifications: A and B: × 100; C: × 200.
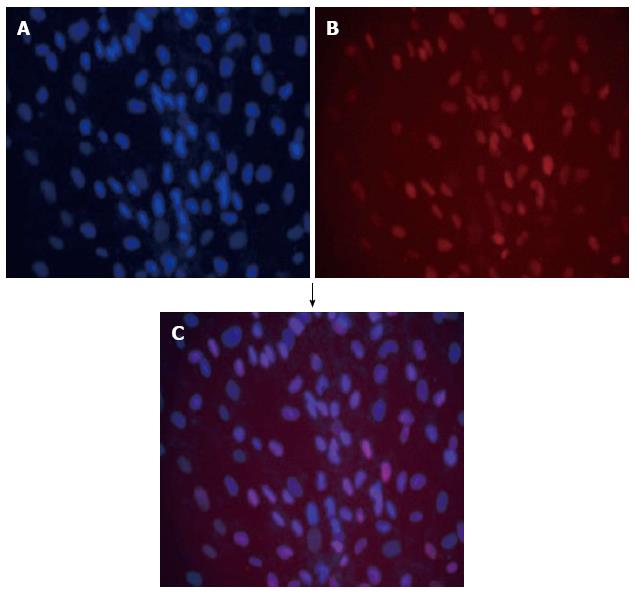
Figure 5 Double immunofluorescence of immortalized hepatocytes stained with DAPI (blue) (A) together with monoclonal antibody anti-SV40T revealed with texas red-antibody conjugate (red) (B); Blue and red fluorescence merged as purple (C).
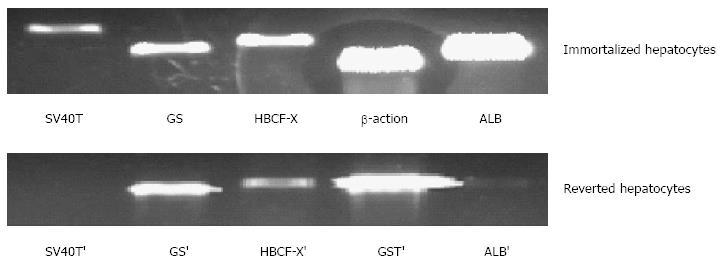
Figure 6 Expression of liver-specific genes associated with liver function in immortalized and reverted cells.
Lanes 1 to 5, simian virus 40 large T antigen (SV40T) and SV40T’, glutamine synthetase) (GS and GS’, human blood coagulation factor X (HBCF-X) and HBCF-X’, β-action and GST’ (glutathione-S-transferase), human albumin (ALB) and ALB’, respectively.
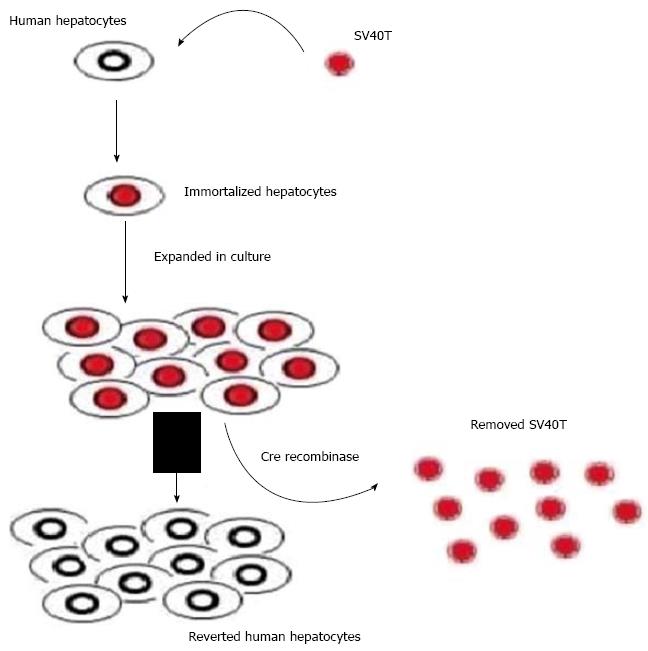
Figure 7 Scheme of reversible immortalization.
The primary hepatocytes were immortalized by transfer of SV40Tag. After expansion of the immortalized cells, Cre/loxP recombination was performed to remove the oncogene (SV40T) and cells reverted to their preimmortalized state.
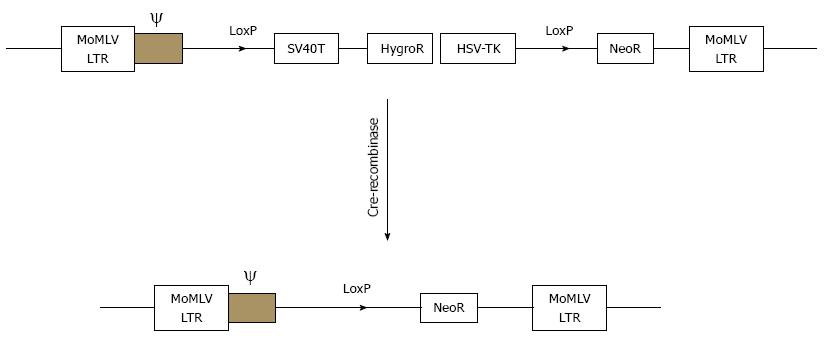
Figure 8 Schematic drawings of the integrating component of retrovitral vector SSR#69 before and after cre-recombination.
SSR#69 contains hygromycin B resistance gene (Hyg R) as a positive selectable marker and the herpes simplex virus thymidine kinase gene (HSV-TK) as a negative selectable marker. The SV40T, Hyg R and HSV-TK genes are flanked by two LoxP sites.
- Citation: Meng FY, Liu L, Yang FH, Li CY, Liu J, Zhou P. Reversible immortalization of human hepatocytes mediated by retroviral transfer and site-specific recombination. World J Gastroenterol 2014; 20(36): 13119-13126
- URL: https://www.wjgnet.com/1007-9327/full/v20/i36/13119.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i36.13119