Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Aug 21, 2014; 20(31): 11000-11011
Published online Aug 21, 2014. doi: 10.3748/wjg.v20.i31.11000
Published online Aug 21, 2014. doi: 10.3748/wjg.v20.i31.11000
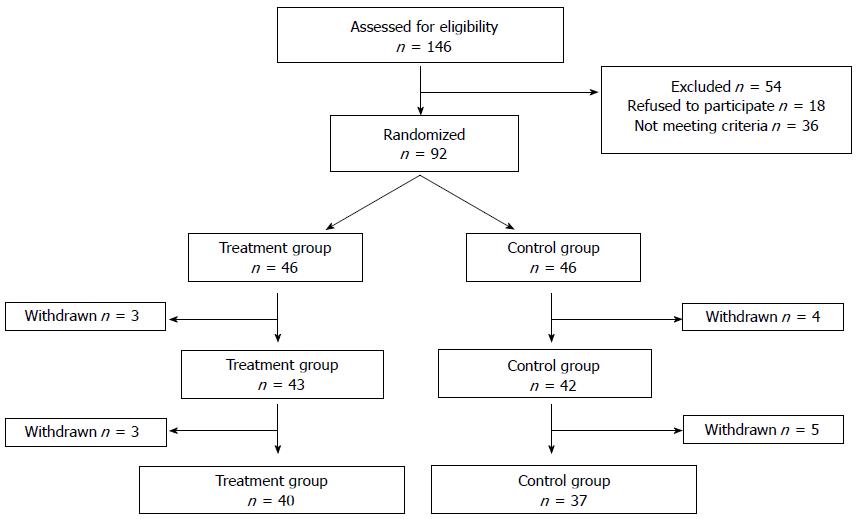
Figure 1 Flow chart of the trial.
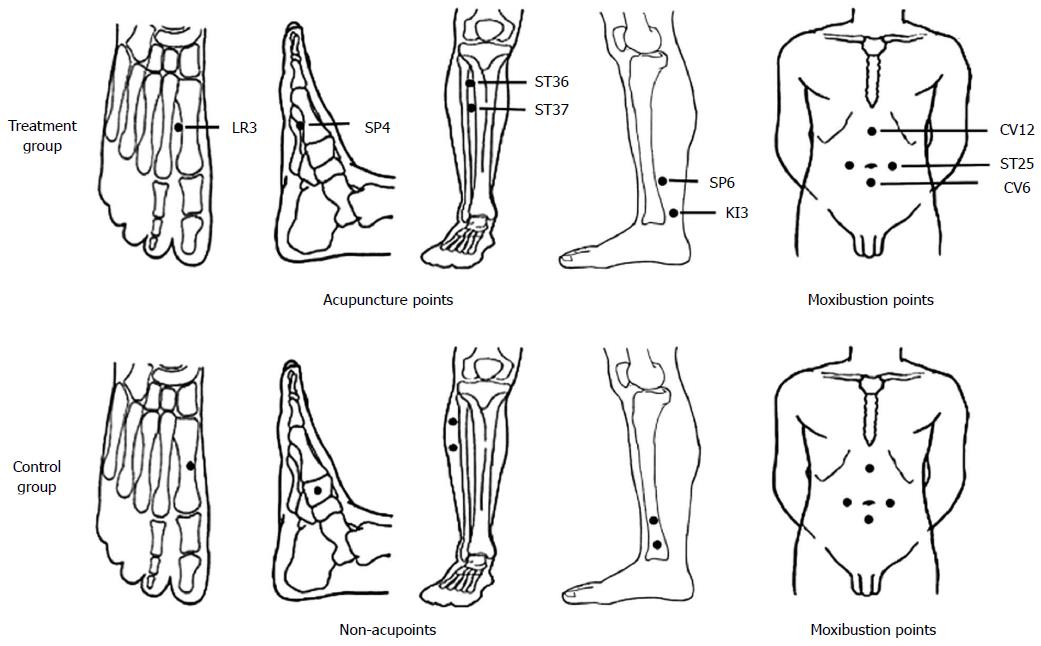
Figure 2 Locations of acupoints and non-acupoints used in this trial.
ST: Stomach; CV: Conception vessel; SP: Spleen; LR: Liver; KI: Kidney.
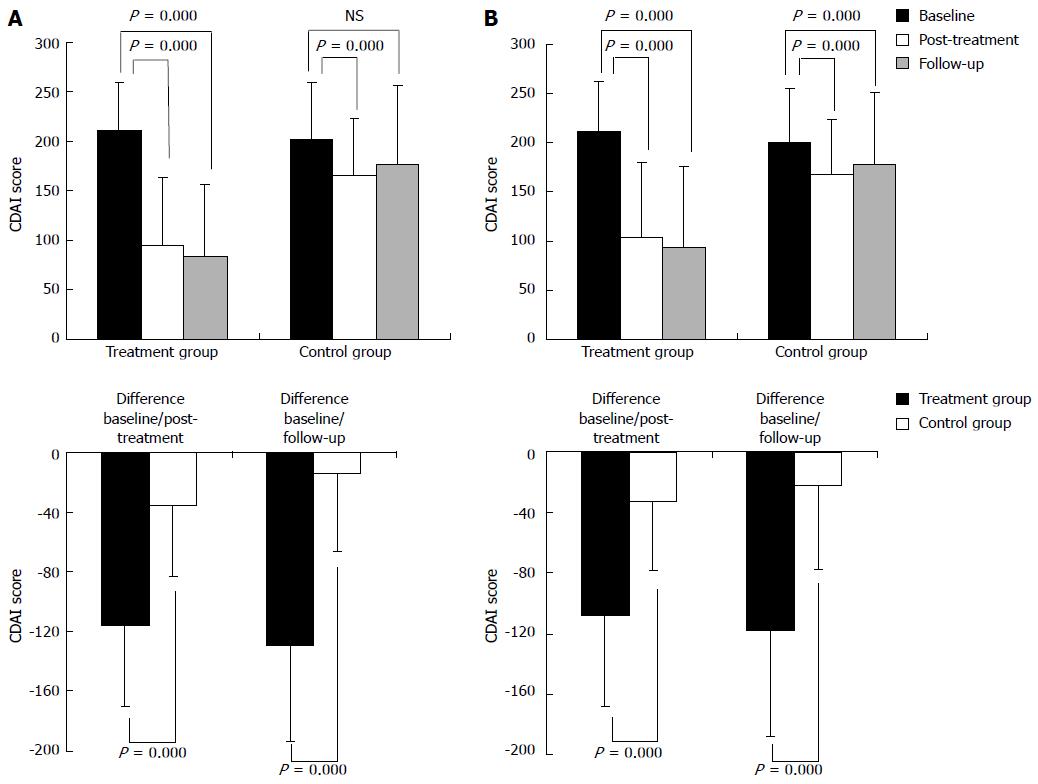
Figure 3 Main outcome measures (Crohn’s disease activity index score).
A: PP analysis; B: Intention-to-treat analysis; CDAI: Crohn’s disease activity index.
- Citation: Bao CH, Zhao JM, Liu HR, Lu Y, Zhu YF, Shi Y, Weng ZJ, Feng H, Guan X, Li J, Chen WF, Wu LY, Jin XM, Dou CZ, Wu HG. Randomized controlled trial: Moxibustion and acupuncture for the treatment of Crohn’s disease. World J Gastroenterol 2014; 20(31): 11000-11011
- URL: https://www.wjgnet.com/1007-9327/full/v20/i31/11000.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i31.11000