Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Aug 7, 2014; 20(29): 9716-9731
Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9716
Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9716
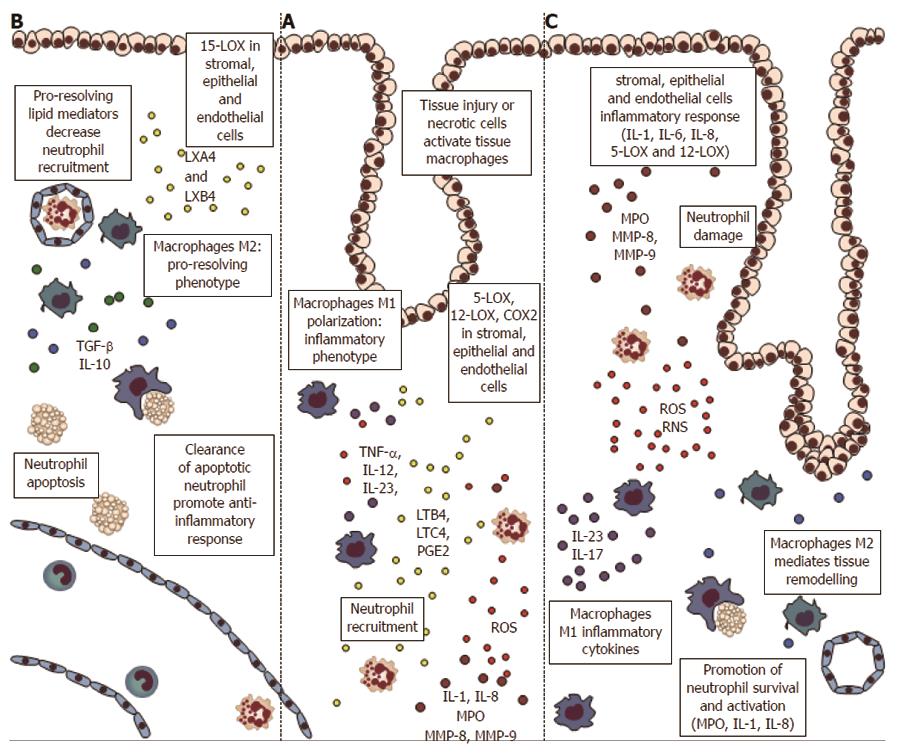
Figure 1 Inflammatory cells and proteins in the early phases of colorectal carcinogenesis.
A: Inflammation and necrosis lead to monocytes recruitment and macrophages M1 polarization, with establishment of an inflammatory microenvironment and cytokines release [tumor necrosis factor α (TNFα), interleukin (IL)-12, IL-23]. Stromal, epithelial, and endothelial cells express lipooxygenases (5-LOX, 12-LOX), and cyclooxygenases 2 (COX2) proteins, with formation of inflammatory mediators leukotrienes and prostaglandins (i.e., LTB4, PGE2) that drive neutrophils recruitment. Neutrophils, at the site of injury, amplify inflammation through myeloperoxydase (MPO), reactive oxygen species (ROS) and matrix metalloproteinases (MMP); B: If the inflammatory stimulus is switched-off the stromal and epithelial cells expressing 15-LOX drive the formation of pro-resolving mediators lipoxins (LXA4 and LXB4). These lipids block the neutrophils migration and stimulate the phagocytosis of apoptotic neutrophils by macrophages M1. The clearance of neutrophils sustain the switch to M2-phenotype, with secretion of anti-inflammatory cytokines such as IL-10 and transforming growth factor beta (TGFβ); C: If the stimulus is not resolved, the stromal and epithelial cells amplify the inflammatory signals (through IL-1, IL-8, 5-LOX and 12-LOX). In this way neutrophils apoptosis is inhibited, with continuous tissue and DNA damage by MPO, ROS and MMPs. The macrophages M1 support the inflammatory environment and the phagocyte afflux (IL-23 and IL-17), while M2 macrophages cause tissue remodeling.
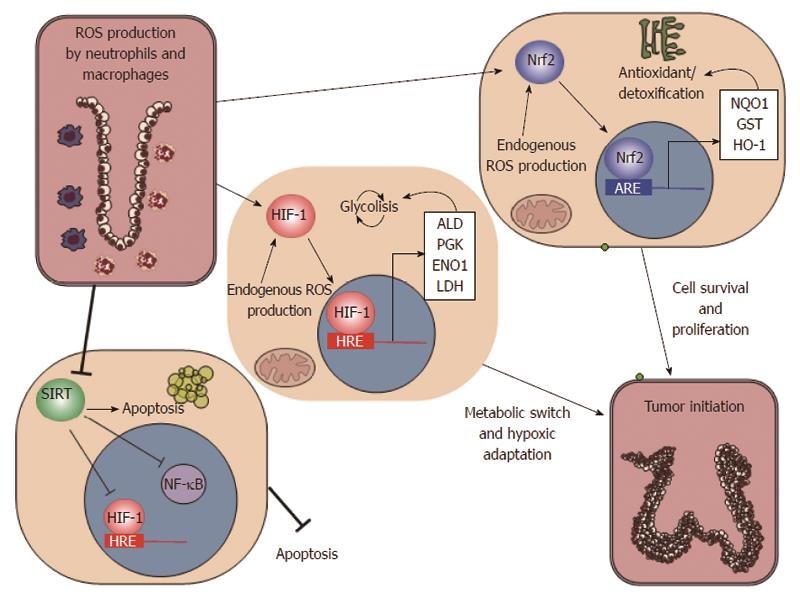
Figure 2 Oxidative microenvironment in the inflammatory milieu of colorectal mucosa.
Inflammation leads to an oxidative microenvironment with consequent modification of cell metabolism. The major players of these changes is hypoxia inducible factor 1 (HIF1), nuclear factor erythroid 2-related factor 2 (Nrf2), and sirtuins (SIRT). HIF1 activation supports the metabolic switch to anaerobial metabolism [fructose-1,6 bisphosphate aldolase (ALD); phosphoglycerate kinase (PGK); enolase 1 (ENO1); lactate dehydrogenase (LDH)]. Nrf2 is involved in the antioxidant defences of epithelial cells [NAD(P)H dehydrogenase (NQO1); glutathione S-transferase (GST); heme oxygenase-1 (HO-1)], while sirtuins affect apoptosis and anti-inflammatory genes.
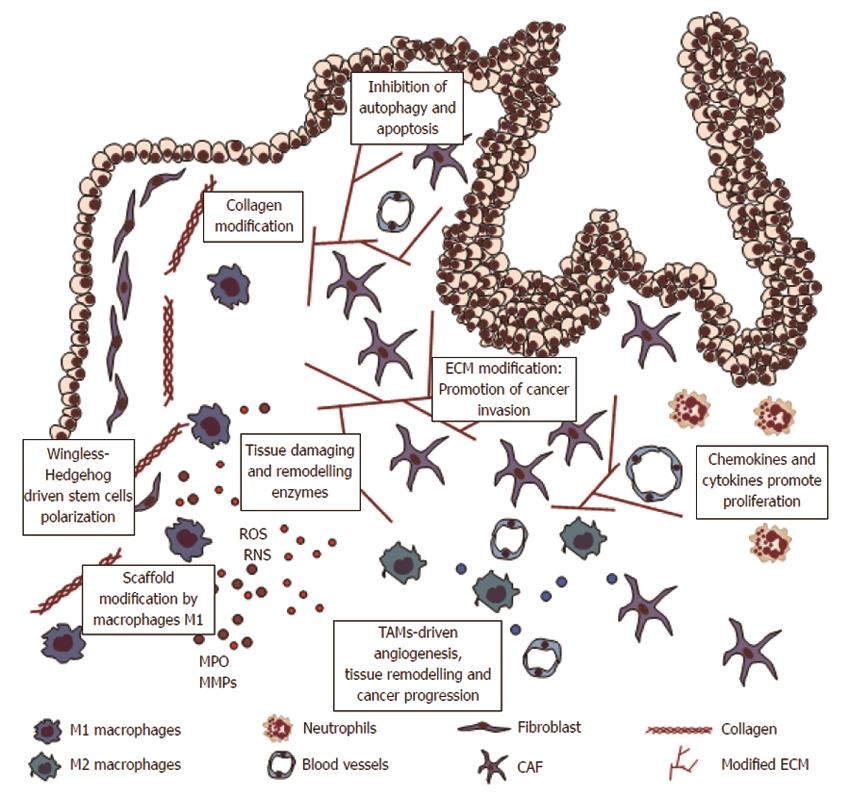
Figure 3 Inflammation and remodelling of colorectal mucosa.
Macrophages and neutrophils cause tissue damage and DNA damage by reactive oxygen species (ROS) formation. Inflammatory cytokines stimulate crypt stem cells proliferation driven by Wingless and Hedgehog. Defects in apoptosis and autophagy systems cause accumulation and proliferation of transformed cells. Indeed, inflammatory cells cause extracellular matrix (ECM) modifications, substaining disassembly of normal tissue architecture, angiogenesis and tumor invasion. CAF: Carcinoma associated fibroblasts; TAMs: Tumor-associated macrophages; MMP: Matrix metalloproteinases.
- Citation: Mariani F, Sena P, Roncucci L. Inflammatory pathways in the early steps of colorectal cancer development. World J Gastroenterol 2014; 20(29): 9716-9731
- URL: https://www.wjgnet.com/1007-9327/full/v20/i29/9716.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.9716