Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Apr 21, 2014; 20(15): 4316-4328
Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4316
Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4316
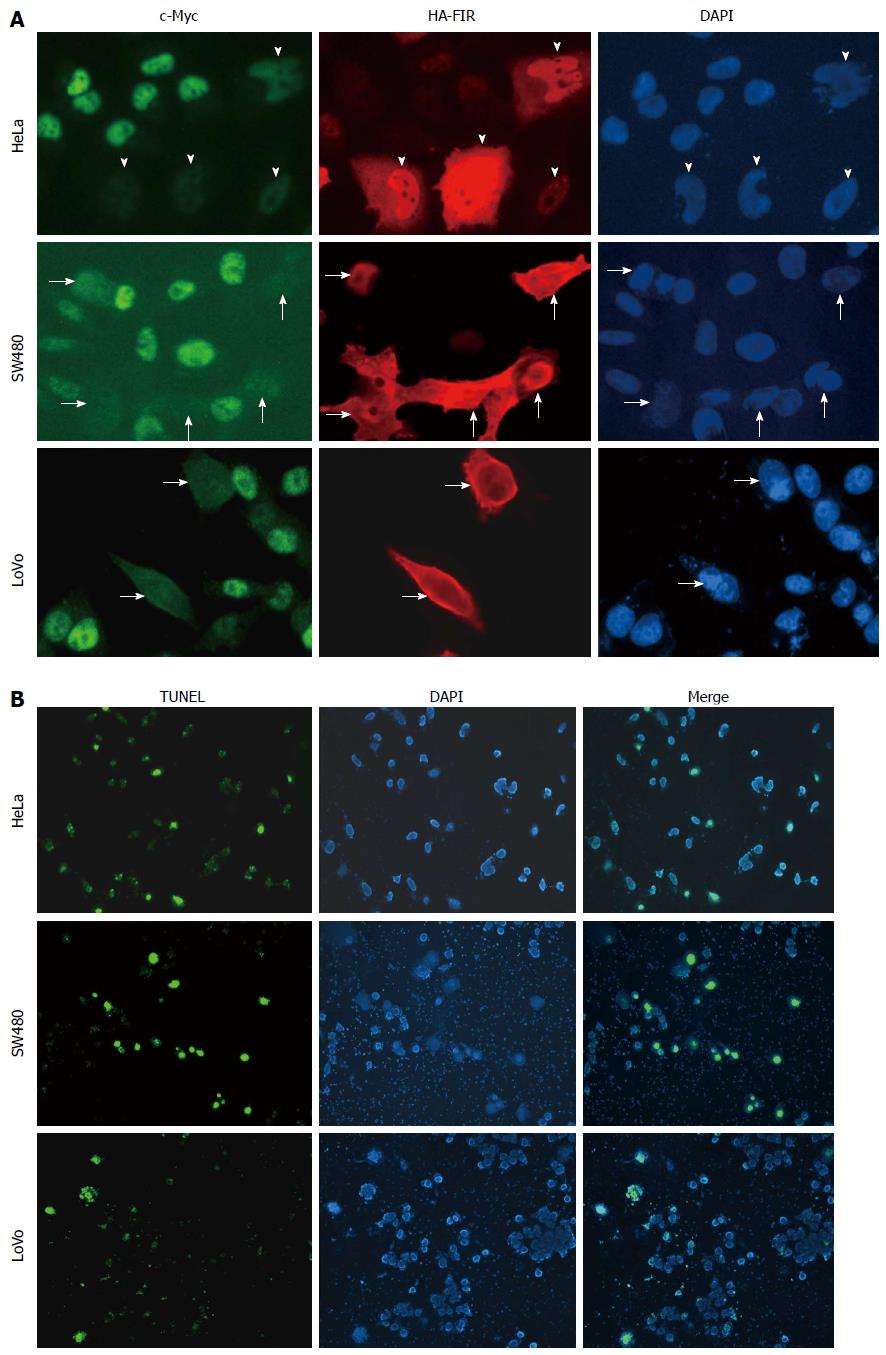
Figure 1 Far up stream element-binding protein-interacting repressor suppresses endogenous c-Myc in SW480 and LoVo cells as well as HeLa cells.
A: 100 fmol of HA-FIR was transfected into the colon cancer cell lines, SW480 and LoVo cells, as well as HeLa cells (cervical squamous cell carcinoma cells) in a 6-well plate. After 48 h transfection, cells were fixed and immunostained against c-Myc (left, green) or HA (middle, red) antibodies. Arrowheads (HeLa), thick arrows (SW480) and thin arrows (LoVo) show the cells in which HA-FIR plasmids were expressed. c-Myc expression (left, green) was markedly reduced in most HA-FIR-expressing cells (middle, red) (indicated by arrowheads and arrows); B: HA-FIR transfected cells were definitively associated with apoptosis as revealed by TUNEL assay in HeLa, SW480 and LoVo cells. Nuclear DNA was stained with DAPI (right, blue). FIR: FBP Interacting Repressor; FBP: FUSE-Binding protein; FUSE: Far Upstream Element.
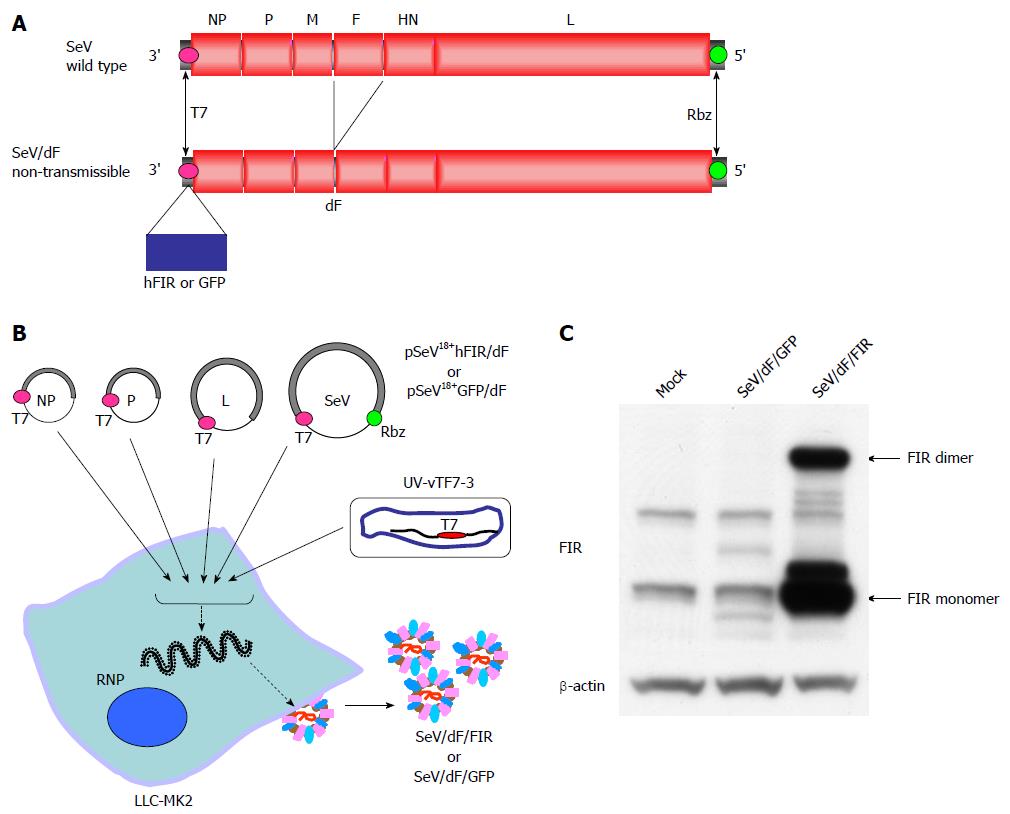
Figure 2 Structures and procedures for generating fusion gene-deficient Sendai virus/dF/far up stream element-binding protein-interacting repressor (SeV/dF/FIR) or Sendai virus/dF/green fluorescent protein vectors (SeV/dF/GFP) from Sendai virus genome RNA.
A: Schematic genome structures of wild-type (SeV) and fusion gene-deficient (SeV/dF; non-transmissible) vector carrying the human FIR (hFIR) gene or jellyfish green fluorescent protein (GFP). The open reading frame or the FIR or GFP gene was inserted with the SeV-specific transcriptional regulatory signal sequences. T7; T7 promoter, Rbz; hepatitis delta virus ribozyme sequence; B: Schematic representation of the procedure for generating the fusion gene-deficient SeV/dF/FIR or SeV/dF/GFP. SeV/dF/FIR or SeV/dF/GFP virus particles were propagated using fusion protein-expressing packaging cells (LLC-MK2/F7/A) after preparation in LLC-MK2 cells using the four plasmids driven by a recombinant vaccinia virus expressing T7 RNA polymerase which had been inactivated with psoralen and long-wave UV light (UV-vTF7-3); C: SeV/dF/FIR virus vectors were infected into HeLa cells and whole cell proteins were extracted for western blot analysis. SeV/dF/FIR expresses FIR proteins. FIR: FBP Interacting Repressor; FBP: FUSE-Binding protein; FUSE: Far Upstream Element; SeV: Sendai virus; NP: Nucleoprotein; P: Phosphoprotein; L: The catalytic subunit of the polymerase large protein forms a ribonucleoprotein complex (RNP) vector and was transfected separately with the SeV RNA. See Materials and Methods.
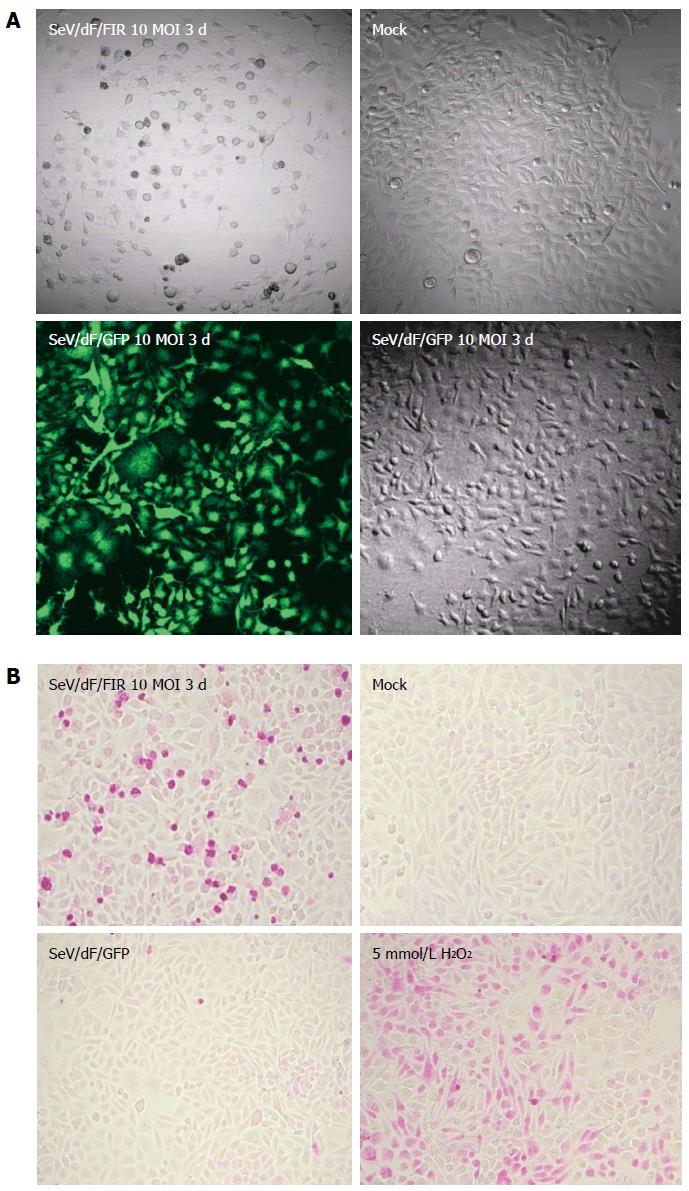
Figure 3 High efficiency of Sendai virus/dF/green fluorescent protein vectors to HeLa cells and Sendai virus/dF/green fluorescent protein vectors indicates significant cell growth inhibition with apoptosis.
A: HeLa cells were infected with a 10 MOI of SeV/dF/FIR virus vector and cultured in DMEM for 3 d (72 h). The same amount of 10 MOI SeV/dF/GFP was also used to infect the control. As shown in the left lower panel, SeV/dF/GFP infected almost 100% cells (green). Under these conditions, SeV/dF/FIR significantly inhibited cell growth as shown in the left upper panel. Mock and 10 MOI SeV/dF/GFP showed no cell growth inhibition as seen in the right upper and lower panels; B: HeLa cells infected by 10 MOI SeV/dF/FIR for 3 d showed significant cell damage revealed by the APO Percentage apoptosis assay™, compared to mock or the same conditions of SeV/dF/GFP virus infected HeLa cells. 5 mmol/L H2O2 was used as a positive control. FIR: FBP Interacting Repressor; FBP: FUSE-Binding protein; FUSE: Far Upstream Element; SeV: Sendai virus; GFP: Green fluorescent protein; MOI: Multiplicity of infection.
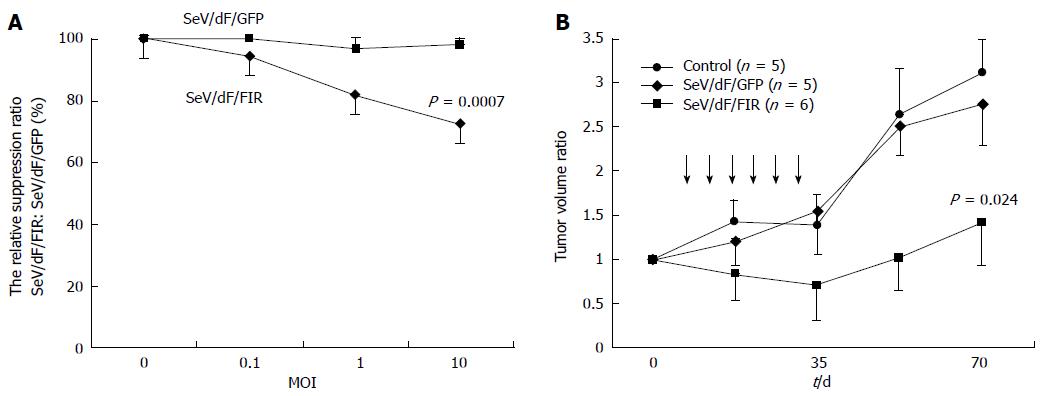
Figure 4 Sendai virus/dF/far up stream element-binding protein-interacting repressor decreased HeLa cell growth in vitro and in a xenograft animal model.
HeLa cells (A) and SW480 cells (B) were infected with 0, 0.1, 1, and 10 MOI of SeV/dF/FIR or SeV/dF/GFP vectors and cell growth was measured by MTS assay (see Materials and Methods). Results are shown as the percentage of cell number at day 0. Points, mean of three separate experiments; bars, SD. Statistical significance was analyzed by Dunnett’s test for multiple comparisons (SeV/dF/FIR vs SeV/dF/GFP, P < 0.007). Two weeks after 5 × 106 HeLa cells were xenografted into the right thigh of Balbc/nu/nu mice, the tumor size was approximately 7-8 mm. 3.0 × 107 CIU of SeV/dF/FIR or SeV/dF/GFP vector were injected directly around the tumor, and the tumor growth was observed and measured every three days as described in Materials and Methods. Results are shown as the ratio of tumor volume compared to the size at day 0. The tumor volume at day 0 of SeV/dF/FIR (n = 6), SeV/dF/GFP (n = 5), and control (n = 5) were 1173.1 + 259.2, 836.0 + 259.2, and 972.2 + 327.0 (average ± SD) mm3, respectively. The average tumor volume at day 0 was estimated as 1 in each experiment. Arrows indicate the injection of SeV/dF/FIR or SeV/dF/GFP vectors. FIR: FBP Interacting Repressor; FBP: FUSE-Binding protein; FUSE: Far Upstream Element; SeV: Sendai virus; GFP: Green fluorescent protein; MOI: Multiplicity of infection.
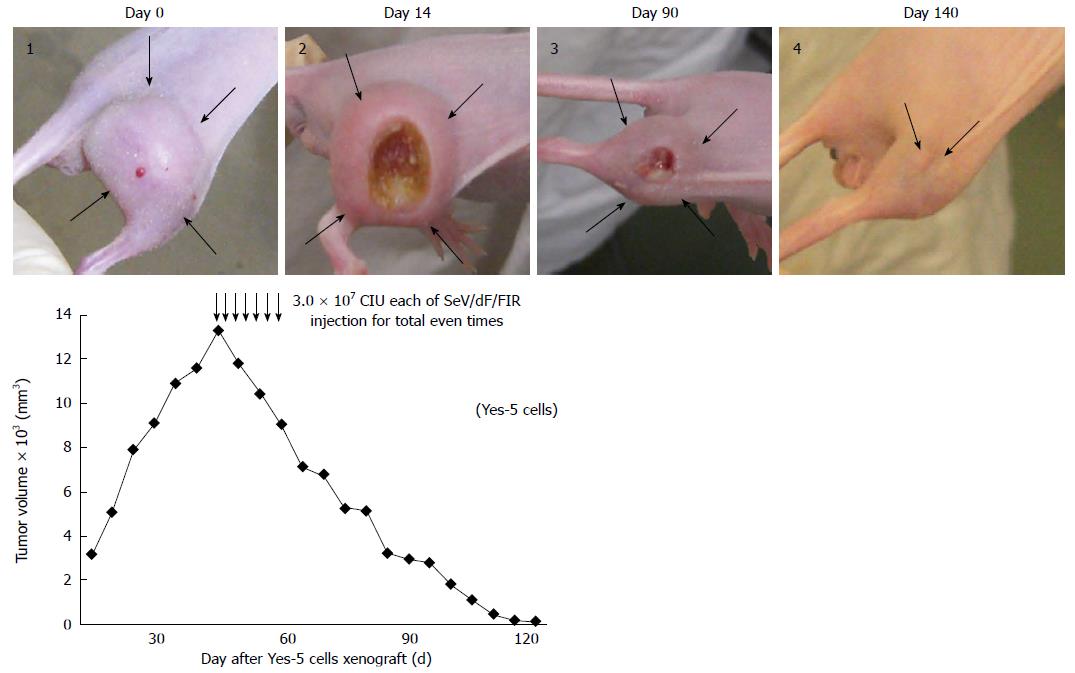
Figure 5 Sendai virus/dF/Far up stream element-binding protein-interacting repressor vector showed anti-tumor activity in a mouse xenograft model.
106 Yes-5 cells were xenografted into the right thigh of Balbc/nu/nu mice, and the tumor size was approximately 15 mm in diameter at Day 0. 3.0 × 107 CIU of SeV/dF/FIR vectors were injected directly around the tumor every two days, seven times in total. Tumor growth was observed and measured every two days as described in Materials and Methods. Ulcer formation was observed in the center of the tumor (day 14 after SeV/dF/FIR injection). Tumor size was significantly diminished with ulcer formation (day 90) and disappeared completely during surveillance (day 140). Thick arrows in the images indicate the tumor margin. Thin arrows indicate the injection of SeV/dF/FIR vectors into the tumor. FIR: FBP Interacting Repressor; FBP: FUSE-Binding protein; FUSE: Far Upstream Element; SeV: Sendai virus; CIU: Cell-infectious units.
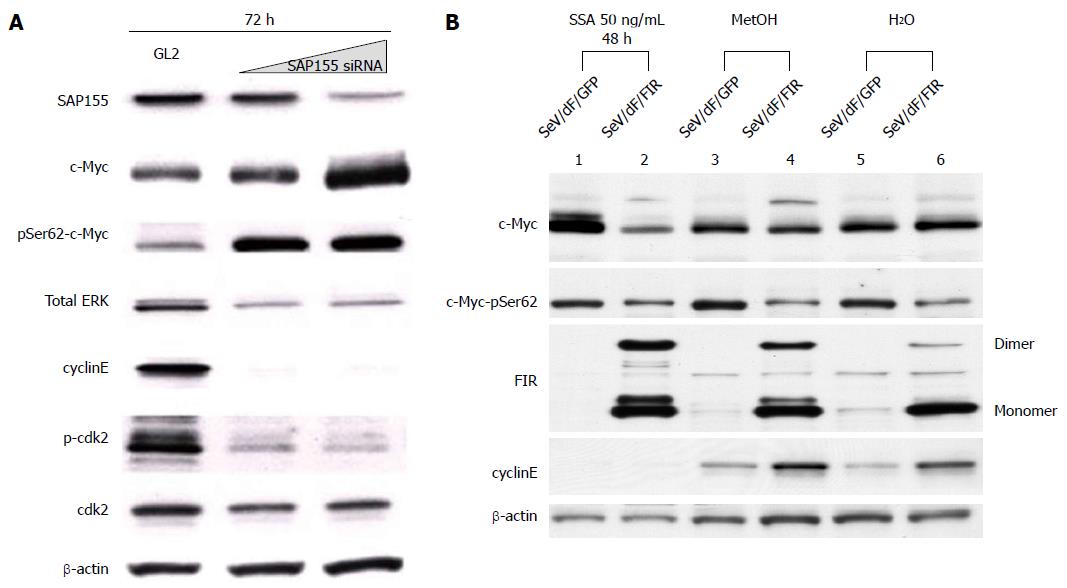
Figure 6 SAP155 siRNA induces c-Myc activation with ErK phosphorylation, but suppresses phosphorylated-cdk2/cyclinE expression.
HeLa cells were treated with SAP155 siRNA for three days (72 h). A: SAP155 siRNA, as well as SSA treatment, increased not only c-Myc expression level, but also c-Myc phosphorylation at both Ser62, but suppressed phosphorylated-cdk2 and cyclinE in a dose-dependent manner. Thus, SAP155 siRNA activates c-Myc potentially via inhibiting endogenous FIR pre-mRNA splicing; B: FIR Sendai virus (SeV/dF/FIR) reversed the cytotoxicity of SSA by suppressing activated endogenous c-Myc. HeLa cells were treated with 50 ng/mL SSA for 48 h with control (MetOH and H2O). 10 MOI of SeV/dF/FIR apparently suppressed activated c-Myc expression, whereas SeV/dF/FIR did not influence basal expression (MetOH or H2O). FIR: FBP Interacting Repressor; FBP: FUSE-Binding protein; FUSE: Far Upstream Element; SeV: Sendai virus; GFP: Green fluorescent protein; MOI: Multiplicity of infection; SSA: Spliceostatin.
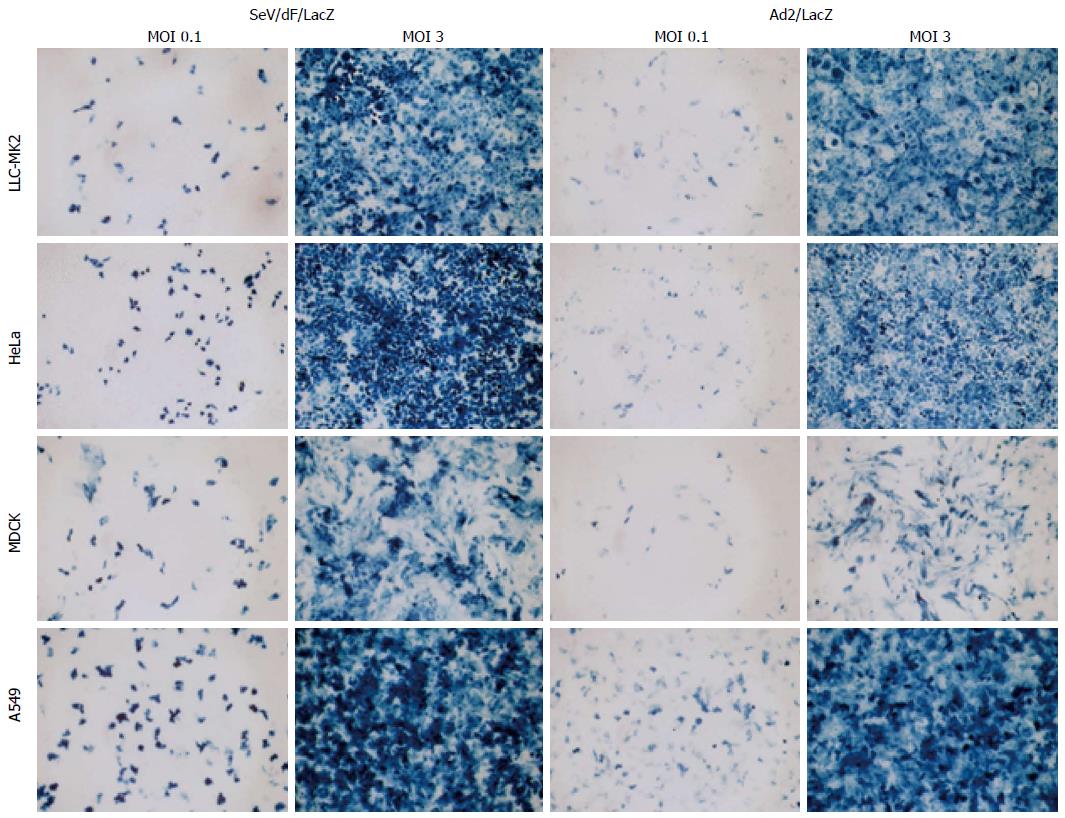
Figure 7 Sendai virus/dF/LacZ transduction efficiency was examined in some human and animal cell lines.
Confluent culture of LLC-MK2 (macaque kidney fibroblasts), HeLa (human adenocarcinoma cells), MDCK (canine kidney cells), and A549 (human lung carcinoma cells) were infected with LacZ expressing SeV vector (SeV/dF/LacZ) at a MOI of 0.1 or 3.0. LacZ expressing Adenovirus vector (Ad5/LacZ) was used as a control. Two days after infection, the cells were stained with X Gal. SeV: Sendai virus.
-
Citation: Matsushita K, Shimada H, Ueda Y, Inoue M, Hasegawa M, Tomonaga T, Matsubara H, Nomura F. Non-transmissible Sendai virus vector encoding
c-myc suppressor FBP-interacting repressor for cancer therapy. World J Gastroenterol 2014; 20(15): 4316-4328 - URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4316.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4316