Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Apr 7, 2014; 20(13): 3418-3430
Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3418
Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3418
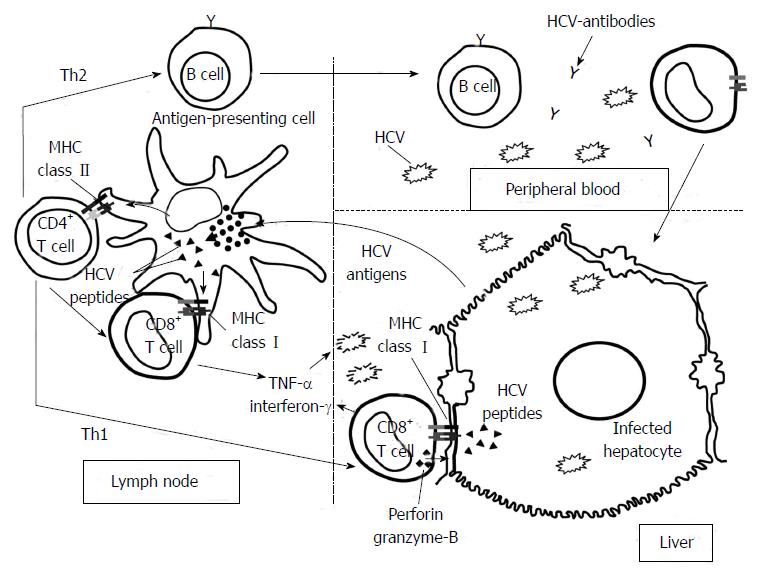
Figure 1 Hepatitis C virus-specific immune response activation.
Graph showing the priming of naïve T cells by professional antigen-presenting cells in the lymph nodes after antigen up-take in the liver. After specific-T cell activation, these cells become effector T helper (Th) and cytotoxic T cells (CTL) and they migrate into the liver. Th2 response regulates B cells while Th1 response controls CTLs effector function. Specifc-CTLs are able to destroy hepatitis C virus (HCV) by cytolytic and non-cytolytic mechanisms.
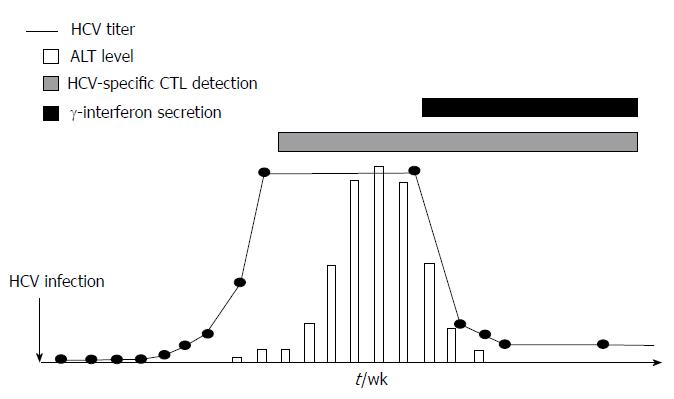
Figure 2 Cytolytic and non-cytolytic mechanisms to eliminate hepatitis C virus by specific cytotoxic T cells.
Scheme showing the hepatitis C virus (HCV) viral load and alanine-amminotransferase (ALT) dynamics after acute HCV infection in relation to appearance of HCV-specific cytotoxic T cells and gamma-interferon secretion. Once HCV-specific cytotoxic T cells are detectable an ALT peak is observed, while when gamma interferon is secreted HCV titers decreased and ALT value becomes normal. CTL: Cytotoxic T lymphocyte.
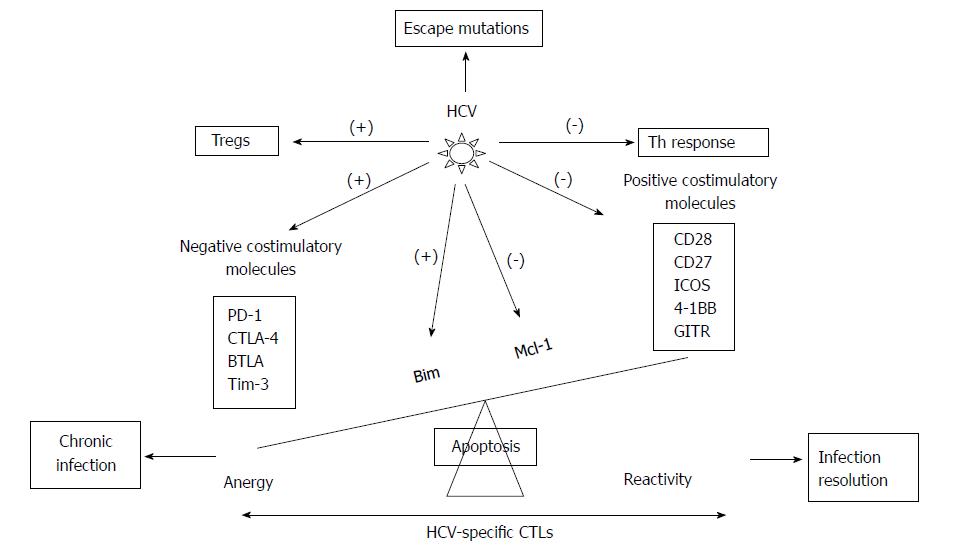
Figure 3 Scheme showing the hepatitis C virus strategies to escape from hepatitis C virus-specific cytotoxic T cells control.
Hepatitis C virus (HCV) modulates the balance between positive and negative co-stimulatory molecules, between pro- and anti-apoptotic molecules, and between Th and Treg cells and develops escape mutations at TCR recognition site. PD-1: Programmed cell death protein 1; CTLA-4: Cytotoxic T-lymphocyte antigen 4; BTLA: B- and T-lymphocyte attenuator; Tim-3: T-cell immunoglobulin domain and mucin domain 3; ICOS: Inducible T-cell Costimulator; GITR: Glucocorticoid induced tumor necrosis factor receptor family related gene; Bim: Bcl-2-interacting mediator; Mcl-1: Myeloid leukemia cell differentiation protein; (-) inhibition; (+) induction.
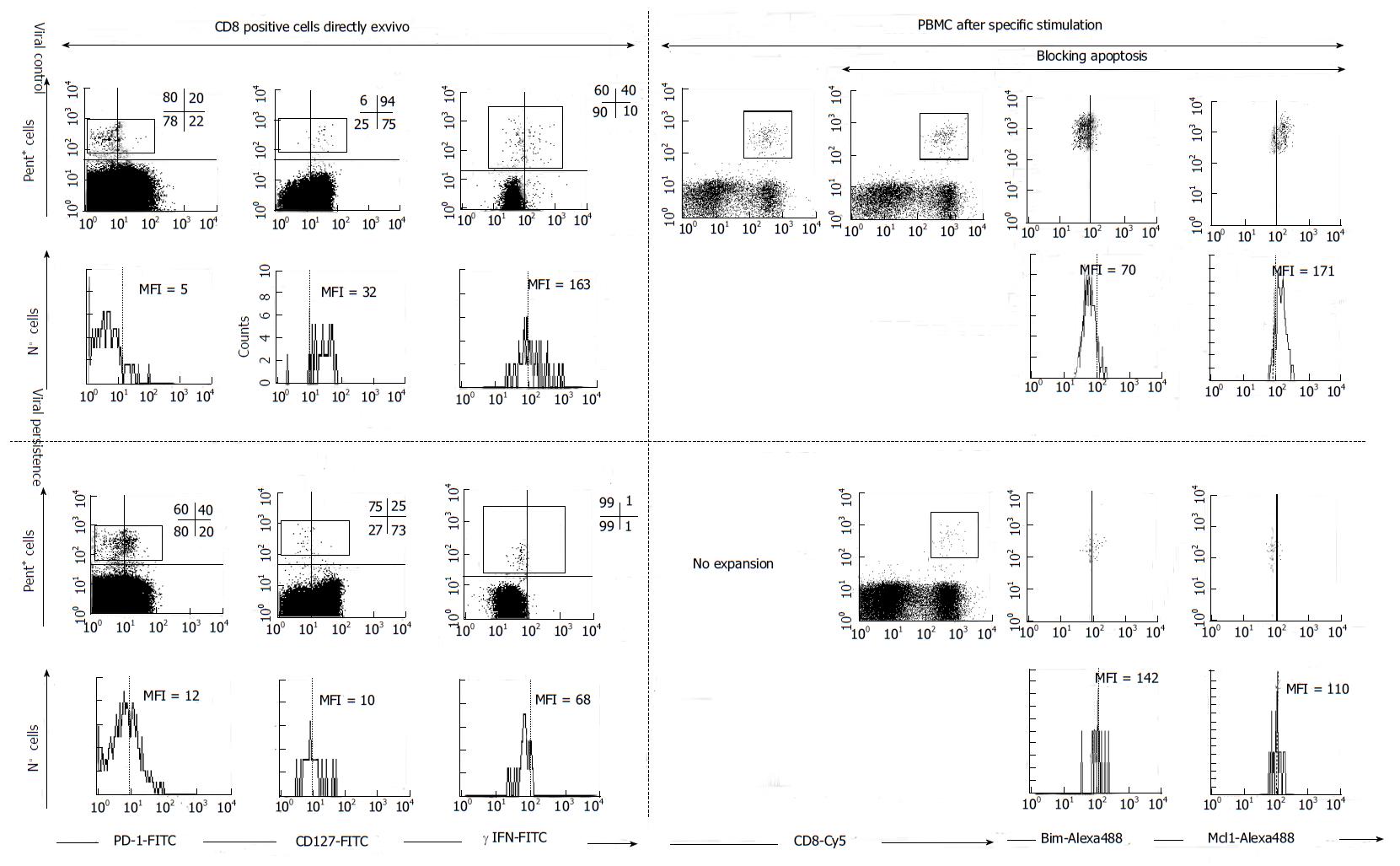
Figure 4 FACS® dot-plots and histograms of hepatitis C virus-specific CD8+ cells from hepatitis C virus patients with different viral control.
Hepatitis C virus (HCV)-specific CD8+ cells were stained with Abs anti γ-IFN, anti-PD-1, anti-CD127, anti-Mcl-1, anti-Bim, anti-CD8 plus pentameric HLA-A2/NS31406 peptide complexes. PD1, γ-IFN and CD127 was analyzed directly ex vivo. Antigen specific proliferation was assessed after blocking or not apoptosis. After expansion Bim and Mcl-1 expression was analyzed. HCV-specific CTLs controlling HCV infection expressed a CD127high, PD-1low, Mcl-1high and Bimlow phenotype while CTLs not controlling HCV displayed the opposite phenotype. MFI: mean fluorescence intensity, Pent: Pe-HLA-A2/NS31406 pentameric complexes. PBMC: Peripheral blood mononuclear cells. PD-1: Programmed cell death protein 1; IFN: Interferon; Bim: Bcl-2-interacting mediator; Mcl-1: Myeloid leukemia cell differentiation protein.
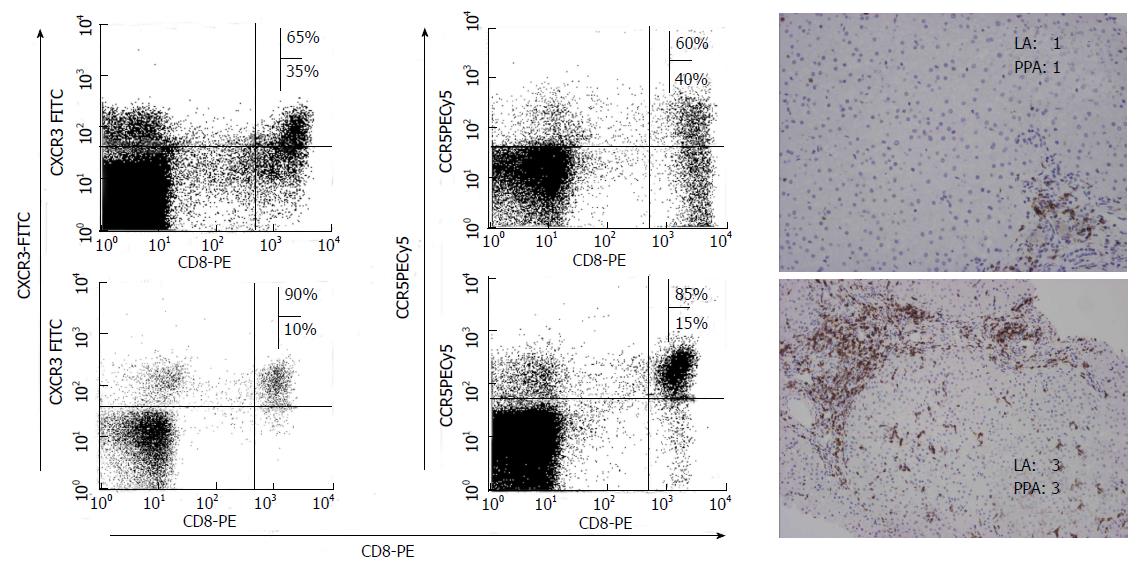
Figure 5 Chemokine receptor expression of liver infiltrating CD8 T cells according to liver damage.
Immunohistochemical CD8 staining of liver samples from patients with different degree of liver inflammation. CCR5 and CXCR3 expression on CD8 T cells from those samples was studied by FACS® analysis after staining with the appropriate mAbs. A positive correlation between CCR5 and CXCR3 expression on intrahepatic total CD8+ T cells and liver inflammation was observed. LA: Lobular activity; PPA: Peri-portal activity according to Metavir Index.
Figure 6 Scheme showing the role of T cell intrahepatic recruitment according to the degree of liver damage and viral control.
In resolved hepatitis C virus (HCV) infection an adequate effector T cell response is attracted to the liver to clear the virus. After that, a memory T cell population is continuously patrolling the liver to keep under control viral traces. Nevertheless, in persistent infection, after HCV-specific T cell failure to control infection, a non-specific inflammatory infiltrate is sequestered into the liver, responsible of the persistent liver damage. CTL: Cytotoxic T lymphocytes.
- Citation: Larrubia JR, Moreno-Cubero E, Lokhande MU, García-Garzón S, Lázaro A, Miquel J, Perna C, Sanz-de-Villalobos E. Adaptive immune response during hepatitis C virus infection. World J Gastroenterol 2014; 20(13): 3418-3430
- URL: https://www.wjgnet.com/1007-9327/full/v20/i13/3418.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i13.3418