Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 28, 2014; 20(12): 3208-3222
Published online Mar 28, 2014. doi: 10.3748/wjg.v20.i12.3208
Published online Mar 28, 2014. doi: 10.3748/wjg.v20.i12.3208
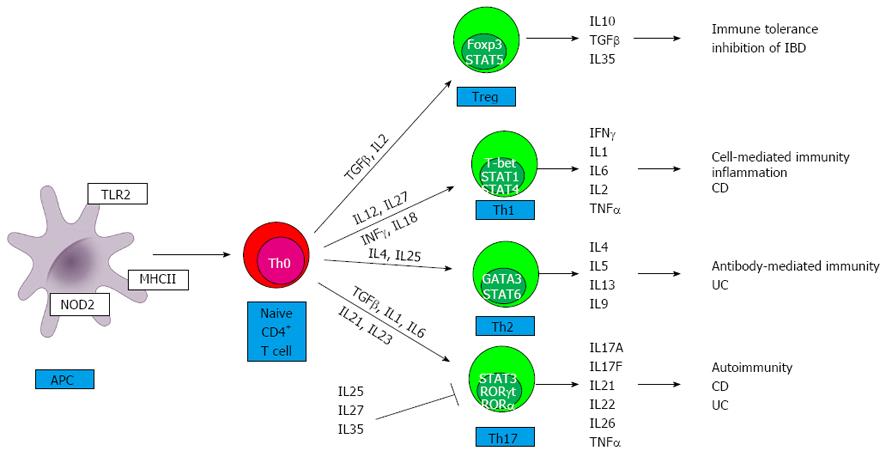
Figure 1 Differentiation of effector T helper and regulatory T cells in inflammatory bowel disease.
Antigen presenting cells (APCs) (i.e., macrophages and dendritic cells) in the lamina propria are increased in absolute number in both forms of inflammatory bowel disease (IBD). First, microbial products (pathogen associated molecular patterns, PAMPs) bind to a group of detection molecules of the innate immune system, called pattern recognition receptors (PRRs). This includes Toll like receptors (TLRs) on cell surface intracellular compartments, and the cytoplasmatic nucleotide-binding oligomerization domain-containing protein (NOD)-like receptors (NLR) family. Stimulation of these receptors induces intracellular signaling cascades, resulting in secretion of large number of cytokines, chemokines, and immunomodulatory factors. APCs interact with T cells by presenting an antigen on the surface of the major histocompatibility complex II (MHCII), which is recognized by the appropriate T cell receptor. The development of T helper (Th)1, Th2, Th17 and regulatory T cells (Treg) subsets from naïve, Th0 cells during primary immune response is mainly determined by this cytokines and chemokines. It is under the control of certain transcription factors: T-box expressed in T cells (T-bet), GATA binding protein (GATA3), retinoid-related orphan receptor (RORγt), RORα, signal transducer and activator of transcription (STATs) and forkhead box P3 (FoxP3). Interleukin (IL)12 is the hallmark cytokine for Th1 cell lines, which produce interferon gamma (IFNγ) and are important for host defense to intracellular pathogens. IL4 promotes differentiation into Th2 cells, which produce IL4, IL5, and IL13 and participate in controlling humoral immunity to extracellular parasites and allergic inflammatory responses. Th17 cells develop from naïve T cells in the presence of transforming growth factor beta (TGFβ), IL23, IL1B or IL6. The effector cytokines IL17A, IL17F, and IL22 play key roles in Crohn’s disease (CD) and ulcerative colitis (UC), and in other autoimmune diseases. TGFβ and IL2 together convert naive T cells into regulatory T cells, which promote self tolerance and prevent autoimmunity. CD is a predominantly Th1 and Th17-mediated disorder, while UC is associated with a Th17 and a modified Th2 cytokine profile.
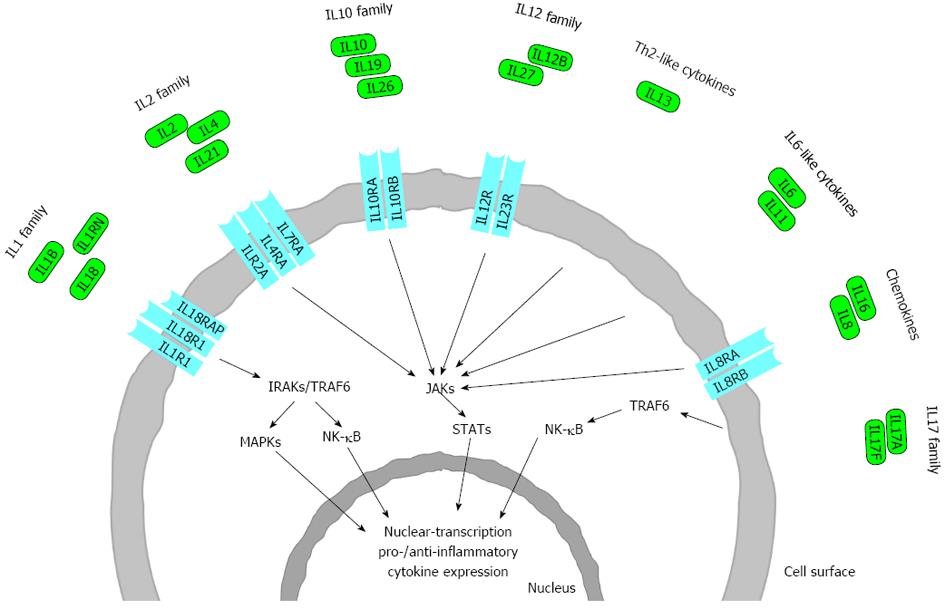
Figure 2 Schematic representation of the interleukin families and receptors involved in the pathogenesis of inflammatory bowel disease.
Only those interleukins (IL) and IL receptors (ILR) are shown where studies have demonstrated association between genes/single nucleotide polymorphisms (SNPs) and disease phenotype. ILs are assigned to each family based on sequence homology and receptor chain similarities or functional properties, considerable overlap between these families exists. Polymorphisms in genes encoding ILs and ILRs have been found to be involved in inflammatory bowel disease. Ligand binding initiates intracellular phosphorylation cascades that are mediated by kinases (i.e., IL1 receptor associated kinase (IRAK); mitogen-activated protein kinase (MAPK); Janus kinase (JAK) and TNF receptor associated factor (TRAF), resulting in signal transduction through certain transcription factors [including signal transducers and activators of transcription (STAT); nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)]. These transcription factors stimulate the expression of a number of proinflammatory and anti-inflammatory cytokine genes involved in inflammatory bowel disease (IBD).
- Citation: Magyari L, Kovesdi E, Sarlos P, Javorhazy A, Sumegi K, Melegh B. Interleukin and interleukin receptor gene polymorphisms in inflammatory bowel diseases susceptibility. World J Gastroenterol 2014; 20(12): 3208-3222
- URL: https://www.wjgnet.com/1007-9327/full/v20/i12/3208.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i12.3208