Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Nov 21, 2013; 19(43): 7680-7695
Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7680
Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7680
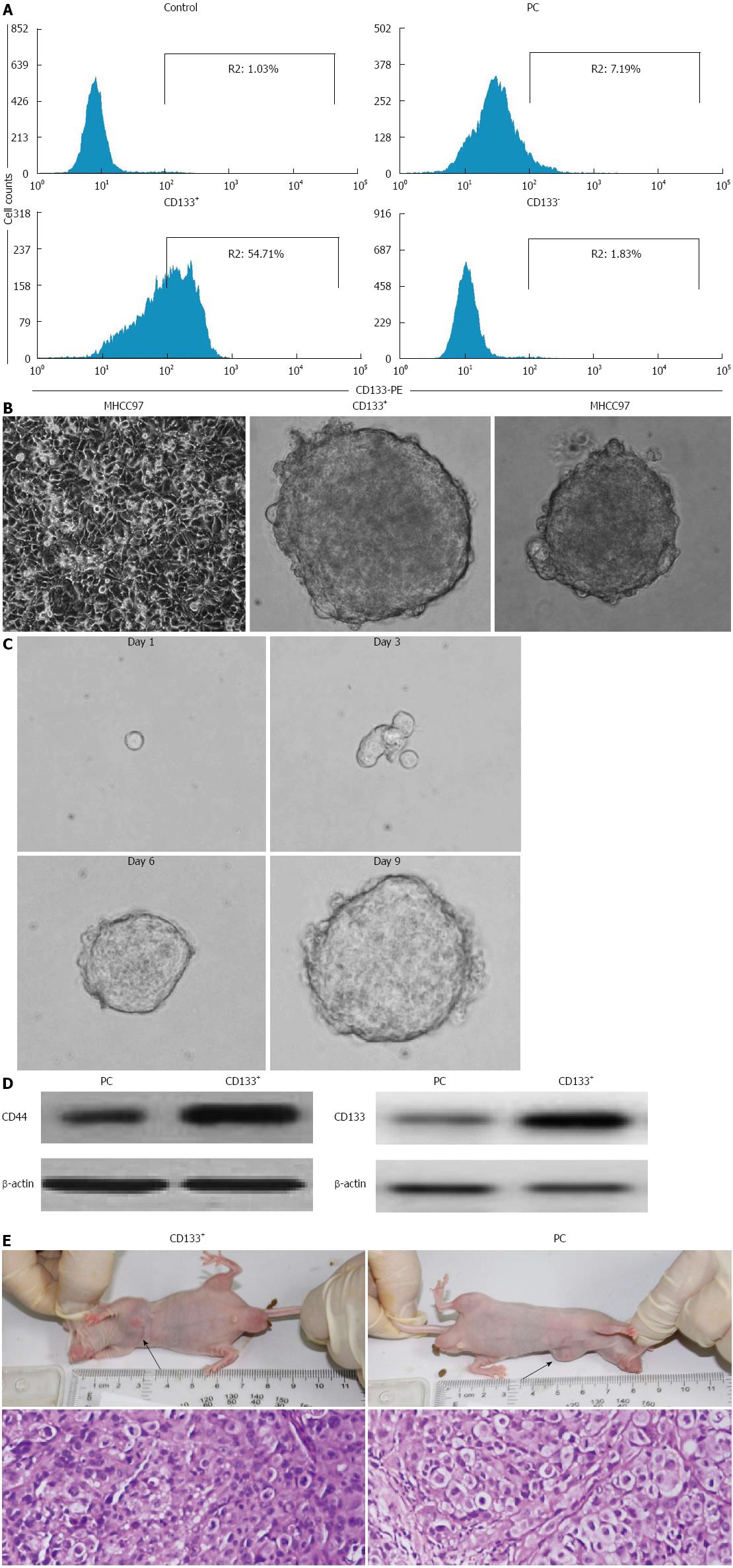
Figure 1 Isolation and characterization of liver cancer stem cells derived from the MHCC97 cell line.
A: Flow cytometry analysis of CD133 expression following sorting. CD133+ cells from MHCC97 cells formed liver cancer spheroids in stem cell-conditioned medium (200 × magnification); B: Anchorage-dependent growth of MHCC97 cells, tumor spheroid formed by CD133+ cells, tumor spheroid formed by parental MHCC97 cells; C: Secondary tumorspheres formed by single cells from dissociated primary liver spheroids (400 × magnification); D: Expression of stem cell surface markers CD44 and CD133 in CD133+ sphere-forming cells (SFCs) and parental cells; E: Hematoxylin-eosin staining revealed similar histological characteristics in tumor xenografts derived from CD133+ SFCs and their parental cells (100 × magnification).
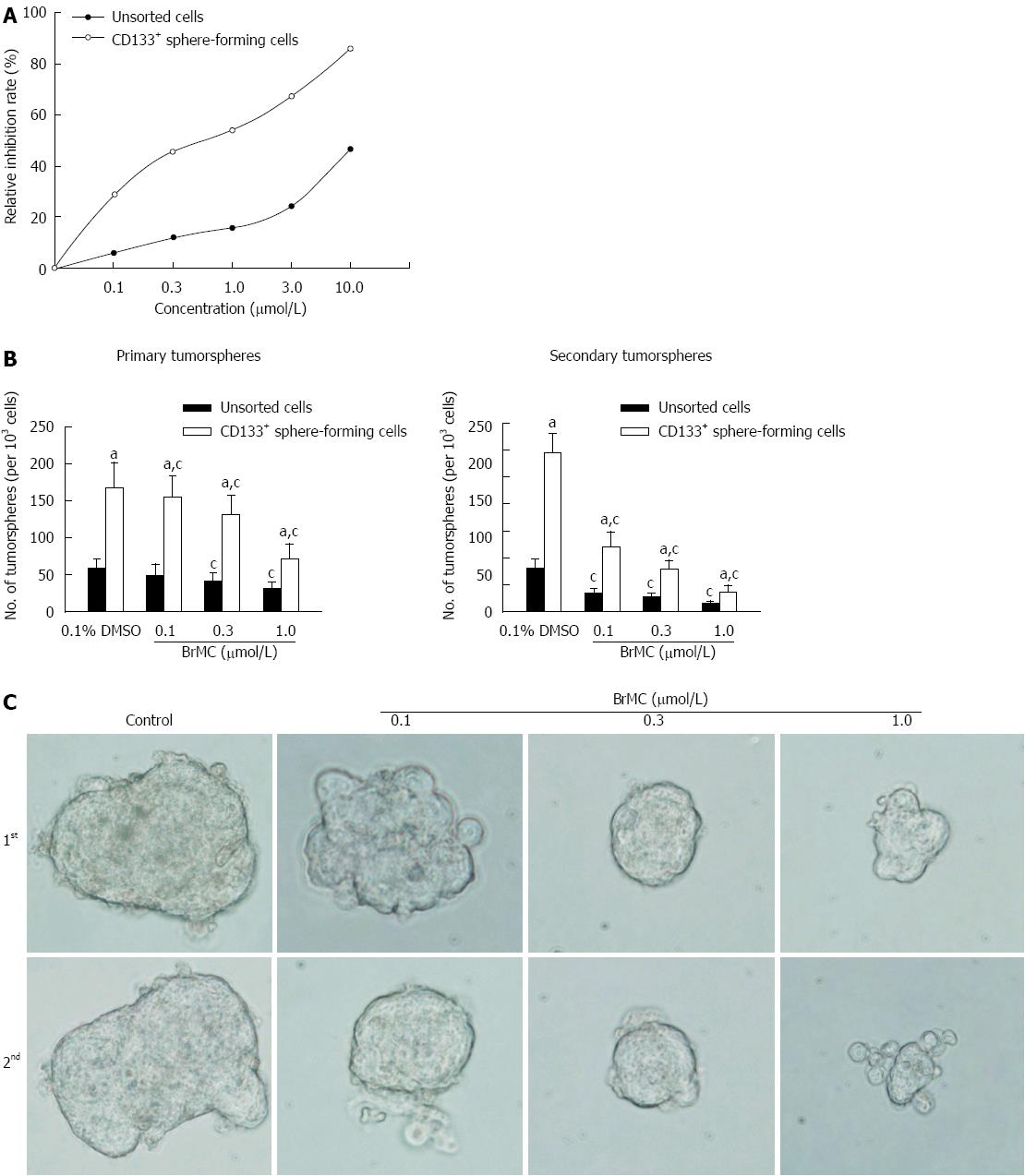
Figure 2 Effects of 8-bromo-7-methoxychrysin on cell proliferation and self-renewal.
8-bromo-7-methoxychrysin (BrMC) inhibited proliferation (A), self-renewal (B and C) of liver cancer stem cells derived from MHCC97 cell line (mean ± SD, n = 3). aP < 0.05 vs unsorted MHCC97 cells treated with corresponding concentrations of BrMC. cP < 0.05 vs corresponding 0.1% DMSO treated group. Tumor sphere morphology is shown as the phase contrast image (400 × magnification).
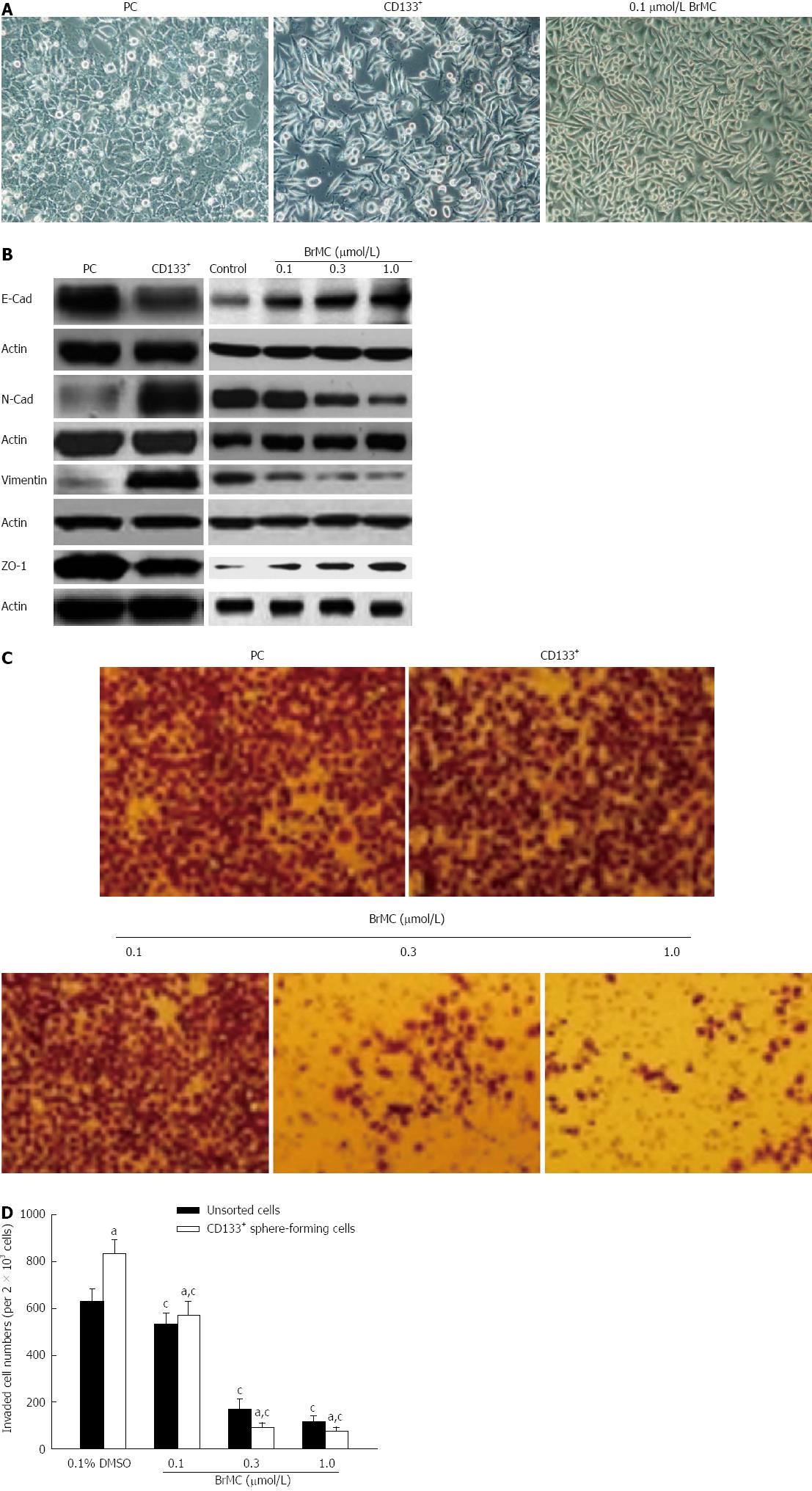
Figure 3 8-bromo-7-methoxychrysin inhibition of liver cancer stem cells.
8-bromo-7-methoxychrysin (BrMC) inhibited epithelial-mesenchymal transition (EMT, A and B) and invasion (C and D) of liver cancer stem cells derived from the MHCC97 cell line (mean ± SD, n = 3). Cell morphological changes associated with EMT are shown as the phase contrast image (200 × magnification). aP < 0.05 vs unsorted MHCC97 cells treated with corresponding concentrations of BrMC, cP < 0.05 vs corresponding 0.1% DMSO treated group.
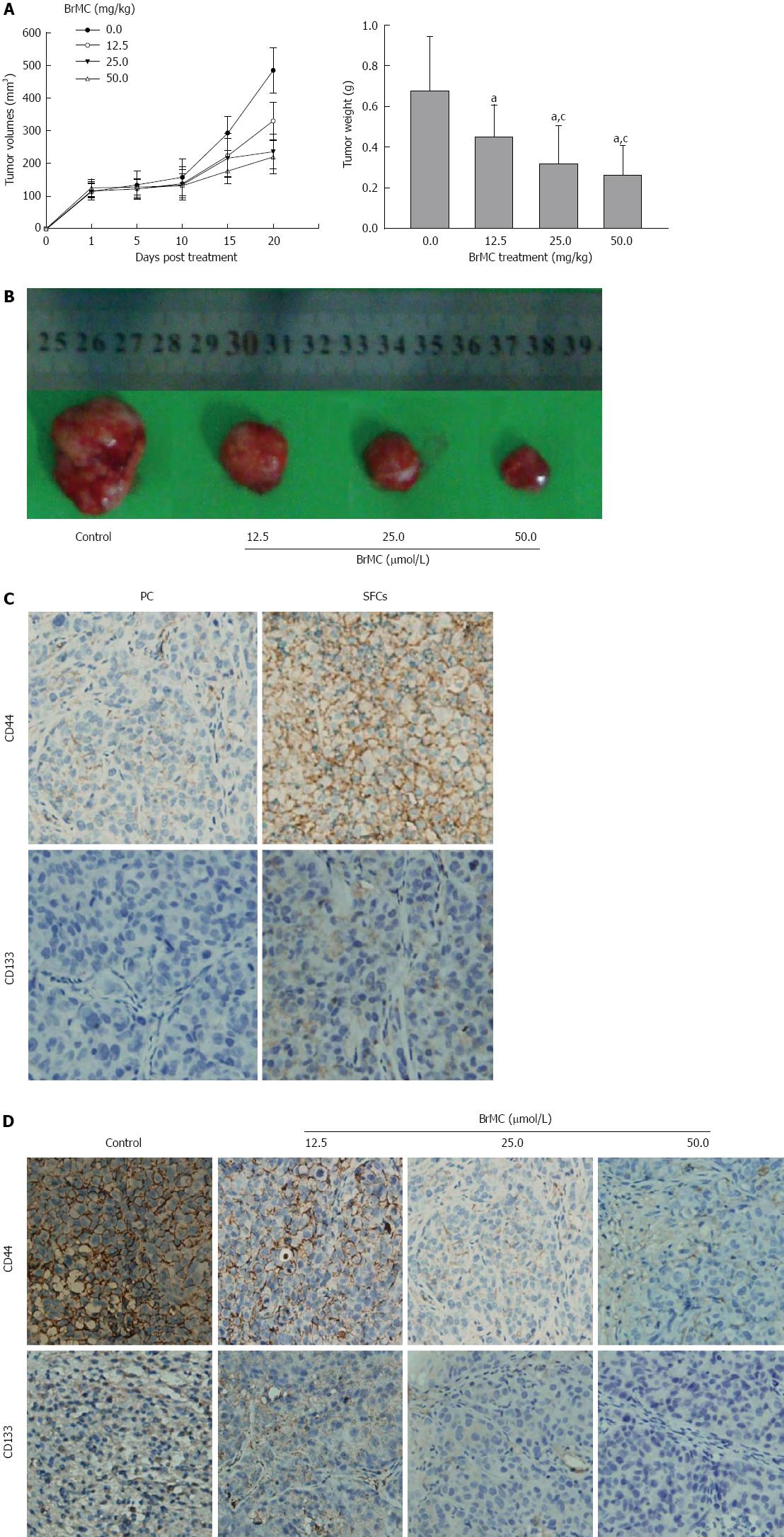
Figure 4 8-bromo-7-methoxychrysin eliminated liver cancer stem cells derived from MHCC97 cell line in vivo.
Effects of 8-bromo-7-methoxychrysin (BrMC) on growth of primary and secondary tumor xenografts derived from liver cancer stem cells (LCSCs) (A and B, mean ± SD, n = 12). aP < 0.05 vs refined olive oil treatment model, cP < 0.05 vs treatment with 12.5 mg/kg BrMC. Immunohistochemical analysis of CD44 and CD133 in LCSC-derived tumors before and after BrMC treatment (C and D).
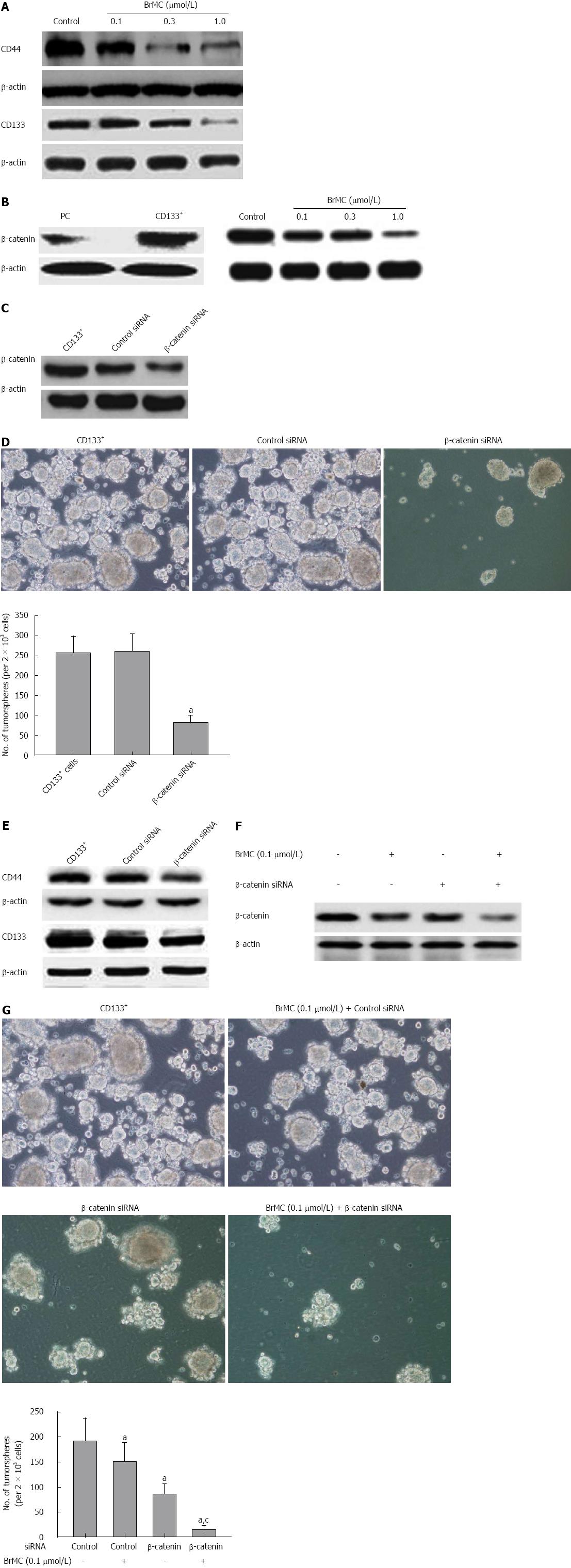
Figure 5 β-catenin siRNA synergized the inhibitory effects of 8-bromo-7-methoxychrysin.
8-bromo-7-methoxychrysin (BrMC) downregulated CD44 and CD133 expression in liver cancer stem cells in a concentration-dependent manner (A). β-catenin was highly expressed in CD133+ sphere forming cells (SFCs) and was downregulated by BrMC treatment (B). β-catenin siRNA decreased the protein level of β-catenin (C) and stem cell markers (E), and significantly inhibited self-renewal capacity (D) of CD133+ SFCs (mean ± SD, n = 3). aP < 0.05 vs CD133+ SFCs or control siRNA transfected CD133+ SFCs. BrMC enhanced β-catenin siRNA induced downregulation of β-catenin expression (F) and inhibition of self-renewal capacity (G) in CD133+ SFCs (mean ± SD, n = 3). aP < 0.05 vs CD133+ SFCs derived from the MHCC97 cell line. cP < 0.05 vs 0.1 μmol/L BrMC or β-catenin siRNA treated CD133+ SFCs.
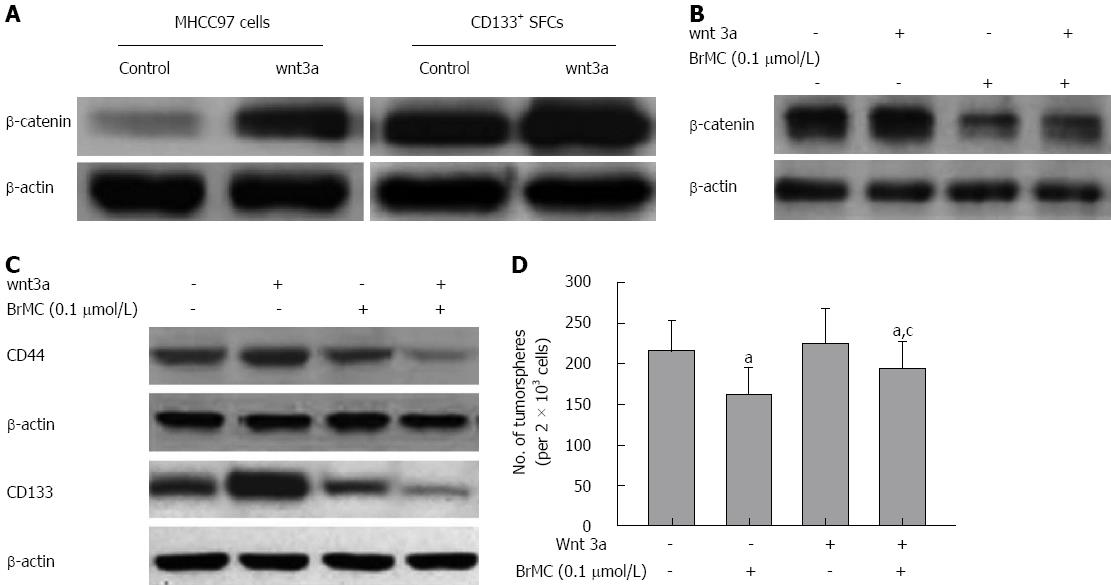
Figure 6 Wnt3a treatment antagonized the inhibitory effects of 8-bromo-7-methoxychrysin.
Wnt3a treatment resulted in an increase in the expression of β-catenin in both liver cancer stem cells (LCSCs) and parental MHCC97 cells (A) and attenuated the effects of 8-bromo-7-methoxychrysin (BrMC) on the expression of β-catenin (B) and stem cell markers (C), and self-renewal capacity (D) of LCSCs derived from the MHCC97 cell line. aP < 0.05 vs CD133+ SFCs, cP < 0.05 vs 0.1 μmol/L BrMC or Wnt 3a alone treated group.
-
Citation: Quan MF, Xiao LH, Liu ZH, Guo H, Ren KQ, Liu F, Cao JG, Deng XY. 8-bromo-7-methoxychrysin inhibits properties of liver cancer stem cells
via downregulation of β-catenin. World J Gastroenterol 2013; 19(43): 7680-7695 - URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7680.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7680