Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Jul 28, 2013; 19(28): 4475-4485
Published online Jul 28, 2013. doi: 10.3748/wjg.v19.i28.4475
Published online Jul 28, 2013. doi: 10.3748/wjg.v19.i28.4475
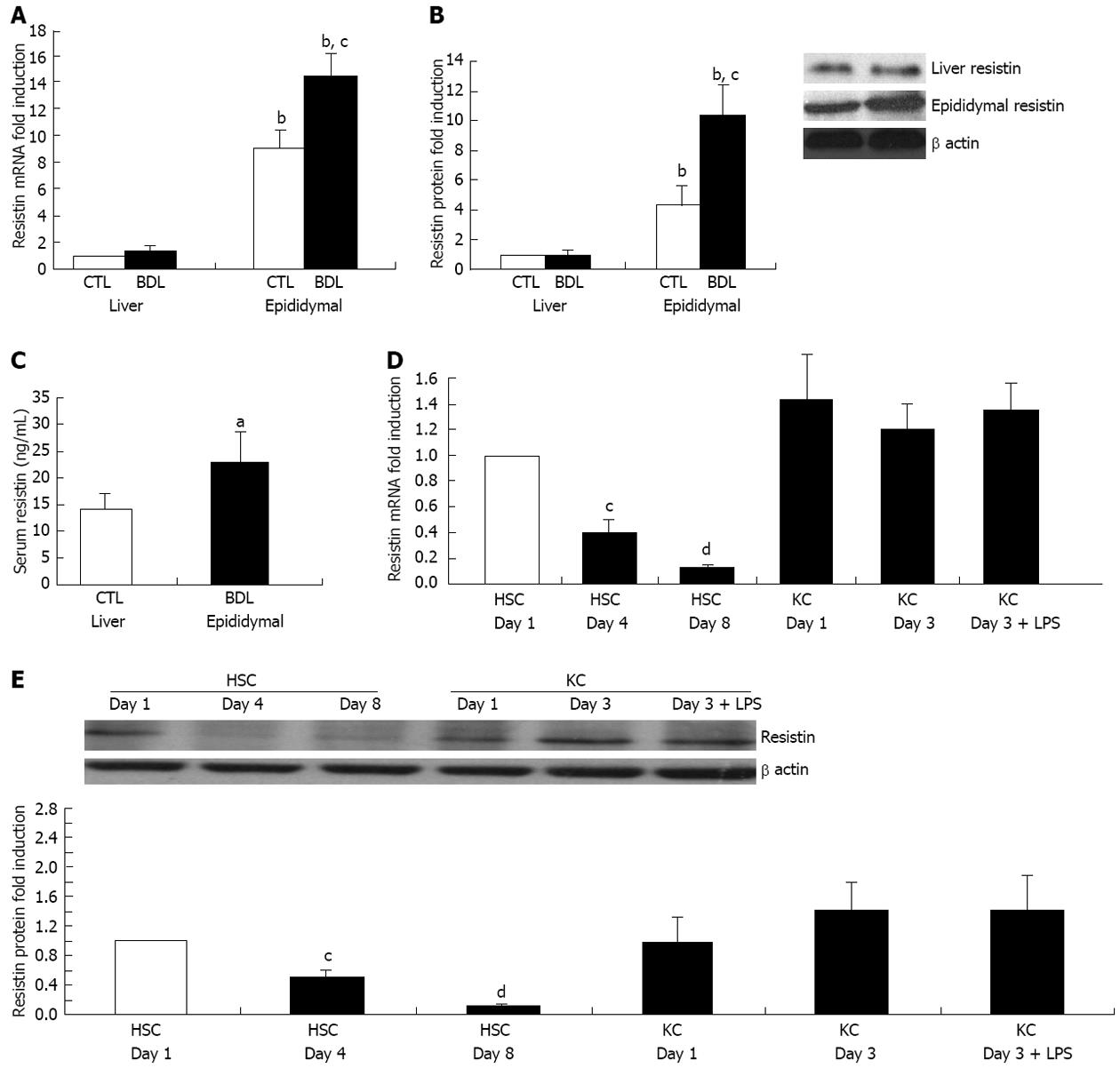
Figure 1 Rat extrahepatic but not intrahepatic resistin is up-regulated in cirrhosis.
For the in vivo animal study, Sprague Dawley rats were subjected to bile duct ligation (BDL) for 4 wk. Liver, adipose tissue (epididymal fat) and serum were collected to determine resistin expression by quantitative polymerase chain reaction (qPCR), immunoblot and enzyme-linked immunosorbent assay. For the cell culture study, rat hepatic stellate cells (HSCs) and Kupffer cells (KCs) were isolated and cultured on plastic. HSC and KC total RNA/protein were extracted at different culture times (day 1, 4 and 8 for HSCs; day 1 and 3 for KCs). One group of KCs at day 2 were treated with Lipopolysaccharide (LPS) (50 ng/mL) for 24 h. qPCR and Immunoblot were performed for quantification of resistin mRNA and protein. β actin was used as an internal control. A: mRNA expression of resistin in liver and epididymal fat; B: Protein expression of resistin in liver and epididymal fat; C: Serum resistin concentrations; D: mRNA expression of resistin in HSCs and KCs on different culture days; E: Protein expression of resistin in HSCs and KCs on different culture days. Results are mean ± SD of at least three independent experiments performed in triplicate. aP < 0.05 and bP < 0.01 increased vs rat liver, HSC control at day 1 or sham rat serum; cP < 0.05 and dP < 0.01 decreased vs liver of control sham rat or HSC control at day 1.
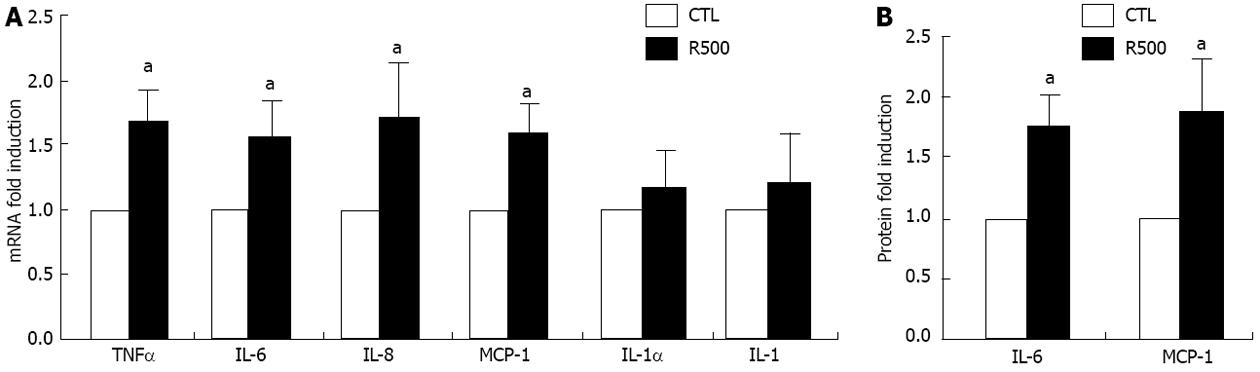
Figure 2 Resistin enhances the expression of tumor necrosis factor α, interleukin 6, interleukin 8 and monocyte chemotactic protein-1 in hepatic stellate cells.
Rat hepatic stellate cells (HSCs) at day 4 were cultured with resistin (500 ng/mL) (R500) for 24 h. Total RNA was extracted and quantitative polymerase chain reaction was performed to quantify mRNA expression. Media were collected and enzyme-linked immunosorbent assay conducted to determine interleukin 6 (IL-6) and monocyte chemotactic protein-1 (MCP-1) protein concentrations. A: mRNA expression of tumor necrosis factor α (TNFα), IL-6, IL-8, MCP-1, IL-1α and IL-1; B: IL-6 and MCP-1 protein levels. Data are expressed as mean ± SD. At least three independent experiments were conducted in triplicate for data analysis. aP < 0.05 vs controls (untreated).
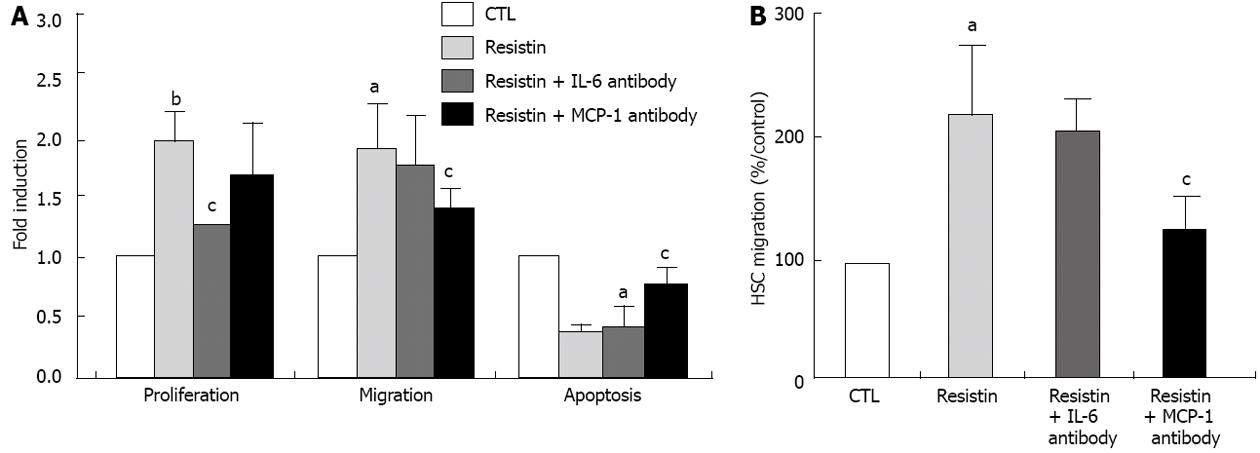
Figure 3 Resistin promotes hepatic stellate cells proliferation and migration but decreases hepatic stellate cells apoptosis in an interleukin 6 and monocyte chemotactic protein-1 dependent mechanism.
BrdU, enzyme-linked immunosorbent assay, Wound Scratch Assay (or Boyden chamber) and annexin V/IP flow cytometry were performed to determine hepatic stellate cells (HSCs) proliferation, migration and apoptosis, respectively. For the interleukin 6 (IL-6) and monocyte chemotactic protein-1 (MCP-1) inhibition experiments, IL-6 and MCP-1 neutralizing antibodies (5 μg/mL and 10 μg/mL, respectively) were added to the culture 1 h before resistin (500 ng/mL) administration. Resistin (500 ng/mL) was added to rat HSCs at day 4 for 24 h. Absorbance was measured and apoptosis assessed. For the migration assay, rat HSCs at day 6 were used. After a scratch wound was made, resistin (500 ng/mL) was added and the cells were cultured for 6 h and photographed. For the Boyden chamber assay, the detailed procedure is described in the Materials and Methods section. A: Resistin promoted HSC proliferation and migration, but inhibited HSC apoptosis, while IL-6 and MCP-1 antibodies reversed the resistin-induced HSC phenotype; B: The Boyden chamber assay confirmed that resistin enhanced HSC migration and MCP-1 neutralization reversed this effect. Results are mean ± SD of at least three independent experiments performed in triplicate. aP < 0.05 and bP < 0.01 vs control (untreated); cP < 0.05 vs resistin treatment alone.
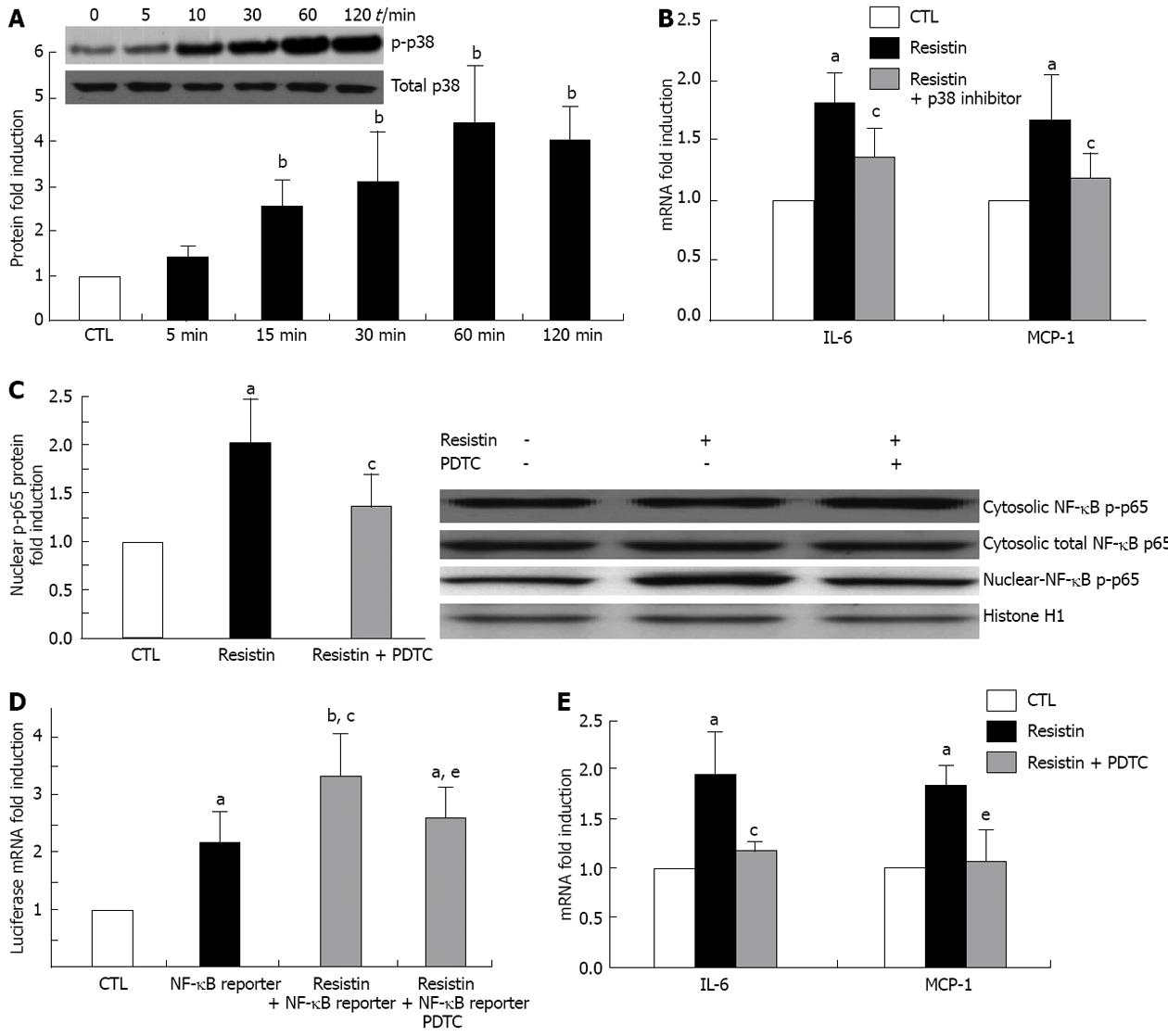
Figure 4 Resistin activates hepatic stellate cells MAPK/p38 and nuclear factor κB p65.
Rat hepatic stellate cells (HSCs) at day 4 were cultured with resistin (500 ng/mL) for 120 min. Cytosolic and nuclear proteins were extracted and Immunoblot performed to quantify p-p38 and nuclear factor κB (NF-κB) p-p65. For NF-κB DNA binding capacity, 3 × NF-κB /Luc reporter was added to the culture for 24 h and Luciferase mRNA quantified by quantitative polymerase chain reaction. For the p-p38 and NF-κB inhibition experiments, SB203580 (20 μmol/L, p-p38 inhibitor) or pyrrolidine dithiocarbamate (PDTC) (100 μmol/L, NF-κB inhibitor) was added 1 h before resistin treatment. Resistin (500 ng/mL) was added to rat HSCs for 24 h. A: p-p38 was enhanced by resistin; B: p-p38 inhibition (24 h) diminished resistin-induced interleukin 6 (IL-6) and monocyte chemotactic protein-1 (MCP-1) increase by HSCs; C: Nuclear p-p65 was increased by resistin exposure and decreased by PDTC (120 min); D: Luciferase mRNA was augmented by resistin and diminished by PDTC (24 h); E: PDTC reversed resistin-induced enhancement of IL-1 and MCP-1 (24 h). Data are expressed as mean ± SD. At least three independent experiments were conducted in triplicate for data analysis. aP < 0.05 and bP < 0.01 vs controls (untreated); cP < 0.05 vs resistin treatment alone or NF-κB reporter treatment alone; eP < 0.05 vs combination of resistin and NF-κB reporter.
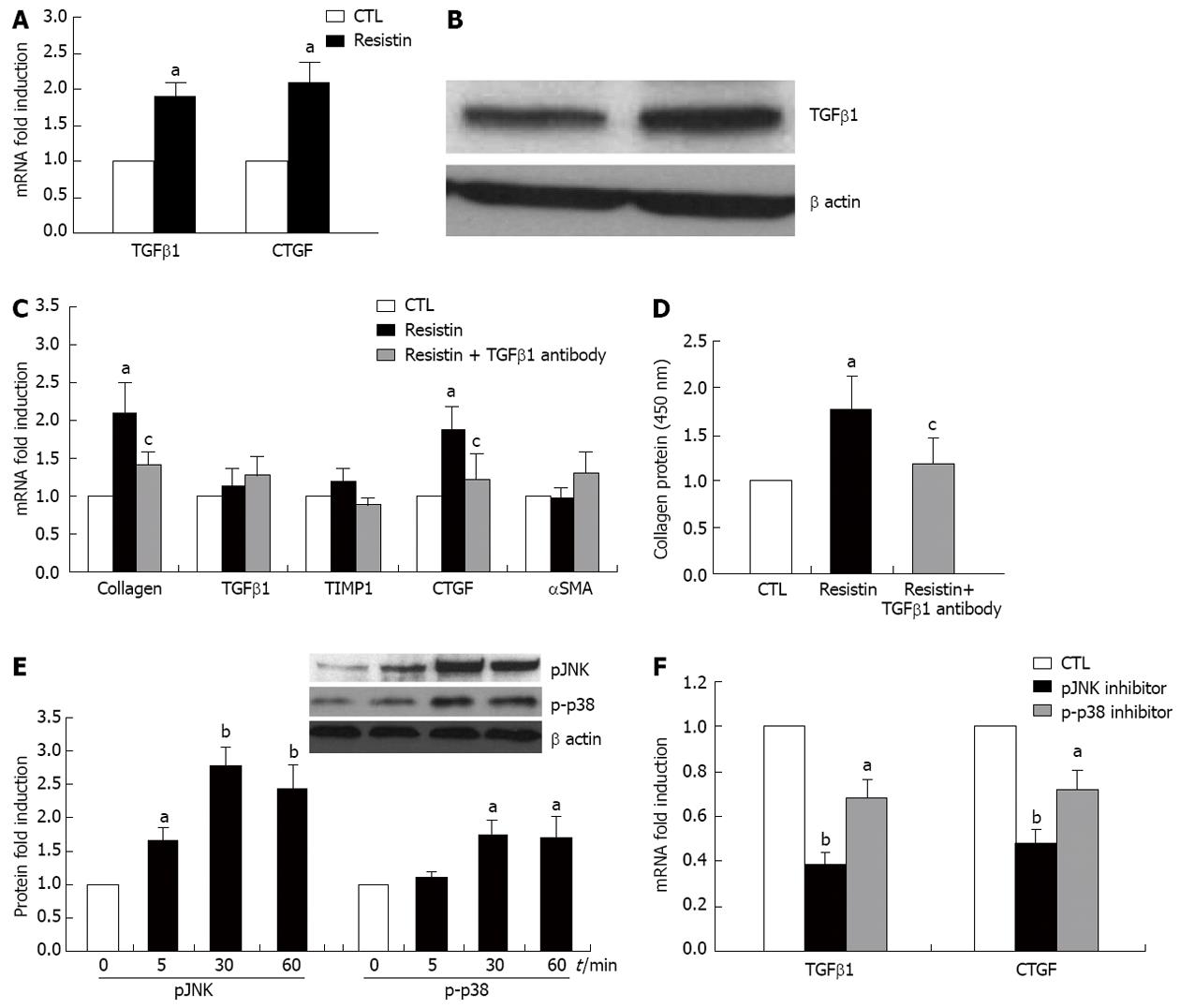
Figure 5 Resistin indirectly enhances hepatic stellate cells collagen I expression through a transforming growth factor β1 dependent mechanism via the action of Kupffer cells.
Rat Kupffer cells (KCs) at day 2 were cultured with resistin (500 ng/mL) for 24 h. Total RNA was extracted and quantitative polymerase chain reaction was performed. Transforming growth factor β1 (TGFβ1) protein expression in KC conditioned medium (KM) was quantified by immunoblotting. Sirius red was used for collagen I protein quantification in the medium. For the KC-hepatic stellate cell (HSC) co-culture experiment, KCs at day 2 were incubated with resistin for 24 h and then washed three times with phosphate-buffered saline, fresh medium was subsequently added to the culture for another 24 h. KM was then transferred to HSCs at day 4 for 24 h. HSC collagen I, TGFβ1, tissue inhibitor of metalloproteinase 1 (TIMP1), connective tissue growth factor (CTGF) and α smooth muscle actin (αSMA) expression were determined. For inhibition experiments, TGFβ1 monoclonal antibody (10 μg/mL), SP600125 (50 μmol/L) and SB203580 (20 μmol/L) were added to KCs for 24 h. A: Resistin promoted KC TGFβ1 and CTGF gene expression; B: Resistin augmented TGFβ1 expression in KM medium; C: HSC collagen I and CTGF mRNA were augmented by resistin conditioned KM reversed by TGFβ1 neutralization (10 μg/mL); D: HSC collagen protein was increased by resistin conditioned KM but reversed by TGFβ1 neutralization (10 μg/mL); E: Resistin increased KC JNK and p38 phosphor-protein; F: pJNK inhibitor (50 μmol/L, SP600125) and p-p38 inhibitor (20 μmol/L, SB203580) partially, but significantly reversed resistin-induced TGFβ1 and CTGF expression by KCs. Data are expressed as mean ± SD. At least three independent experiments were conducted in triplicate for data analysis. aP < 0.05 and bP < 0.01 vs controls (untreated); cP < 0.05 vs resistin treatment alone.
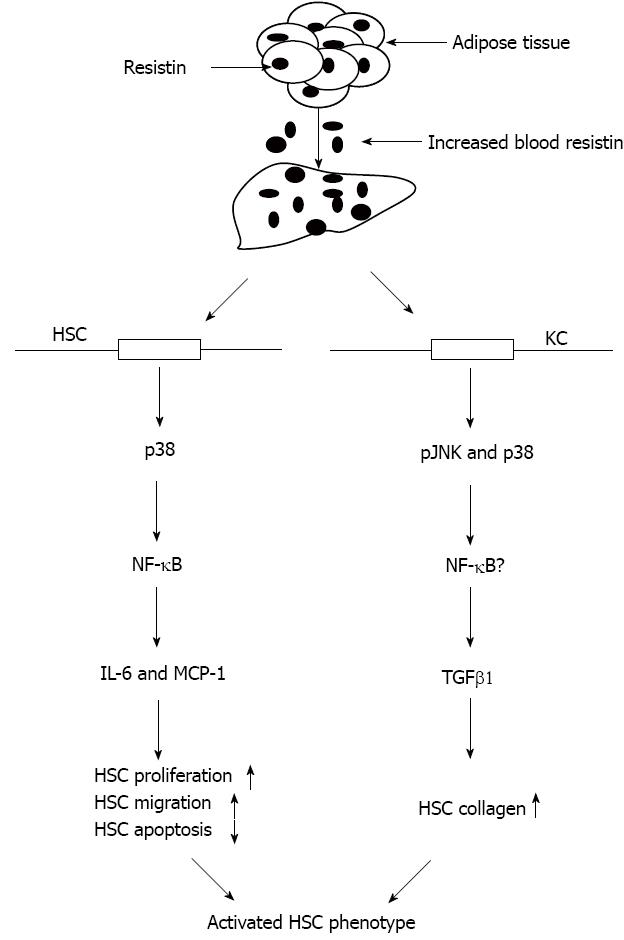
Figure 6 Schematic diagram illustrating a possible mechanism by which resistin potentiates hepatic stellate cells profibrogenic phenotype.
Adipose tissue is a predominant source of resistin expression and secretion in rodents. Increased adipose resistin released into the bloodstream and liver stimulates HSCs to produce increased amounts of pro-inflammatory cytokines and chemokines (IL-6 and MCP-1), leading to enhanced HSC proliferation and migration, but attenuation of HSC apoptosis. In addition, resistin promotes KC TGFβ1 which subsequently activates HSC by up-regulation of collagen I. Therefore, resistin is considered one of the pro-fibrogenic adipocytokines. TGFβ1: Transforming growth factor; NF-κB: Nuclear factor κB; IL-6: Interleukin 6; MCP-1: Monocyte chemotactic protein-1; HSCs: Hepatic stellate cells.
- Citation: Dong ZX, Su L, Brymora J, Bird C, Xie Q, George J, Wang JH. Resistin mediates the hepatic stellate cell phenotype. World J Gastroenterol 2013; 19(28): 4475-4485
- URL: https://www.wjgnet.com/1007-9327/full/v19/i28/4475.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i28.4475