Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 21, 2013; 19(11): 1707-1717
Published online Mar 21, 2013. doi: 10.3748/wjg.v19.i11.1707
Published online Mar 21, 2013. doi: 10.3748/wjg.v19.i11.1707
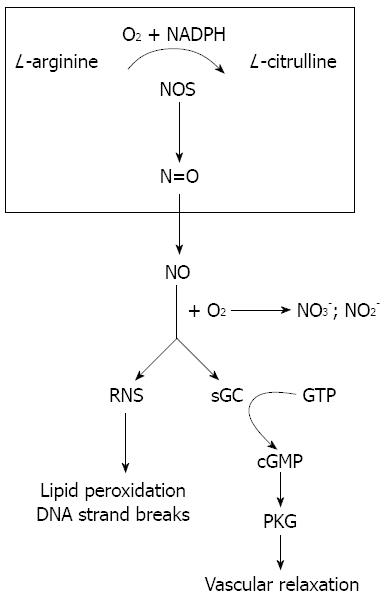
Figure 1 Nitric oxide formation and function.
Nitric oxide synthase (NOS) catalyzes the biosynthesis of nitric oxide (NO) from L-arginine, nicotinamide adenine dinucleotide phosphate (NADPH) and O2•NO freely diffuses into cells where it mediates vascular relaxation by stimulating the cyclic guanosine 3’-5’-monophosphate (cGMP)/cGMP-dependent protein kinase G (PKG) pathway. It also forms reactive nitrogen species (RNS) which leads to many damaging reactions including lipid peroxidation and DNA strand breaks. GTP: Guanosine 5’-triphosphate; sGC: Soluble guanylyl cyclase.
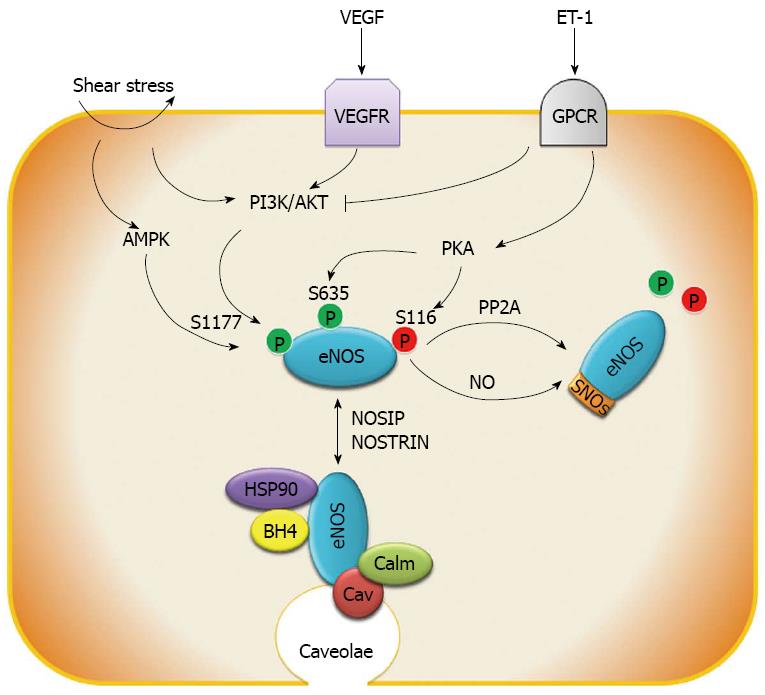
Figure 2 The molecular regulation of endothelial nitric oxide synthase activity.
Endothelial nitric oxide synthase (eNOS) phosphorylation can be triggered by shear stress, vascular endothelial growth factor (VEGF), endothelin 1 (ET-1) and other factors though adenosine monophosphate-activated protein kinase (AMPK), protein kinase B (AKT) and protein kinase A (PKA) pathways, whereas protein phosphatase 2 (PP2A) de-phosphorylates eNOS. In addition, S-nitrosylation (SNOs) by eNOS-derived nitric oxide (NO) inhibits eNOS activity. Endothelial nitric oxide synthase interacting protein (NOSIP) and endothelial nitric oxide synthase trafficking inducer protein (NOSTRIN) regulate the sub-cellular location of eNOS protein between the caveolae and cytoplasm. The principal location of eNOS is in caveolae where its function is inhibited by binding to caveolin (Cav). HSP90, calmodulin (Calm) and tetrahydrobiopterin (BH4) are indispensable proteins and cofactors for catalyzing NO production. PI3K: Phosphatidylinositol-3-kinase; GPCR: G protein-coupled receptor.
- Citation: Hu LS, George J, Wang JH. Current concepts on the role of nitric oxide in portal hypertension. World J Gastroenterol 2013; 19(11): 1707-1717
- URL: https://www.wjgnet.com/1007-9327/full/v19/i11/1707.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i11.1707