Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. May 21, 2012; 18(19): 2334-2343
Published online May 21, 2012. doi: 10.3748/wjg.v18.i19.2334
Published online May 21, 2012. doi: 10.3748/wjg.v18.i19.2334
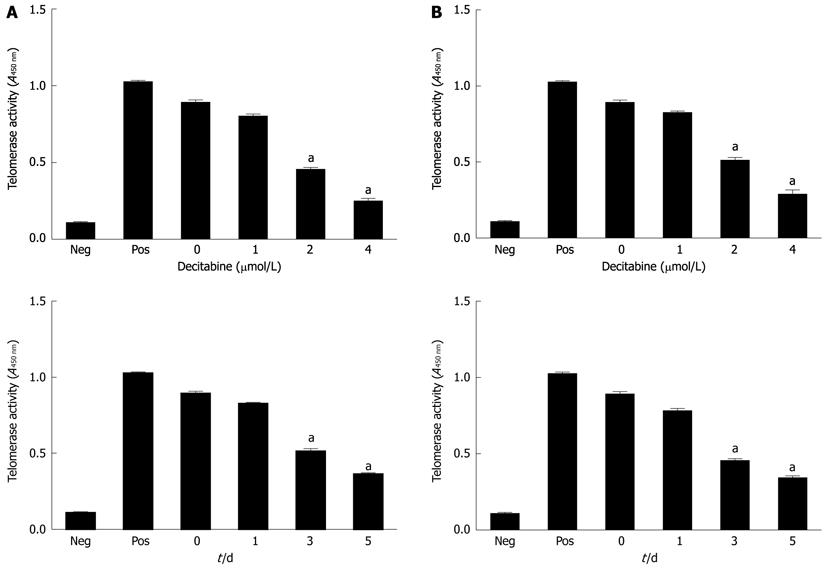
Figure 1 Effect of 5-aza-2’-deoxycitidine on telomerase activity in human hepatocellular carcinoma cell lines SMMC-7721 (A) and HepG2 (B).
Cells were incubated with DAC (1 μmol/L, 2 μmol/L or 4 μmol/L). Cell pellets were collected and subjected to telomeric repeat amplification protocol-enzyme-linked immunosorbent assay. aP < 0.05 (by unpaired Student’s t test). Neg: Negative control; Pos: Positive control; DAC: 5-aza-2’-deoxycitidine. All studies are representative of at least three independent experiments.
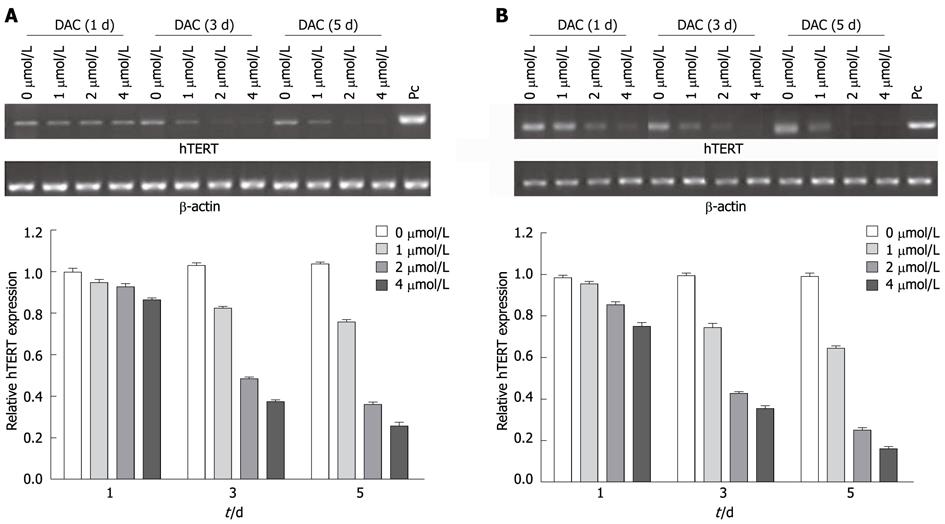
Figure 2 Effect of 5-aza-2’-deoxycitidine on telomerase reverse transcriptase mRNA in hepatocellular carcinoma cell lines SMMC-7721 (A) and HepG2 (B).
Cells were incubated with DAC (1 μmol/L, 2 μmol/L or 4 μmol/L). Cell pellets were collected and subjected to real-time reverse transcription-polymerase chain reaction assay. PC: Positive control; DAC: 5-aza-2’-deoxycitidine; hTERT: Human telomerase reverse transcriptase.
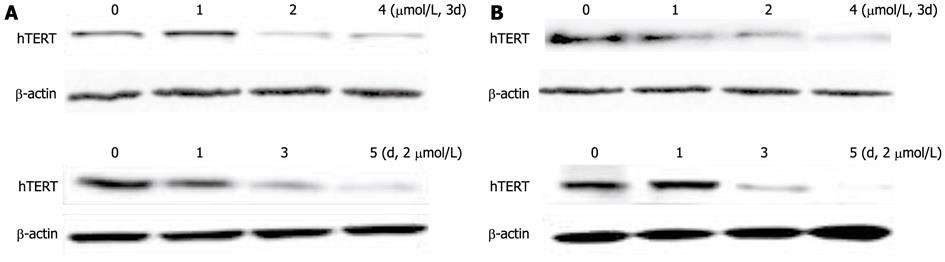
Figure 3 Expression of telomerase reverse transcriptase protein in hepatocellular carcinoma cell lines SMMC-7721 (A) and HepG2 (B) during exposure to 5-aza-2’-deoxycitidine (1 μmol/L, 2 μmol/L or 4 μmol/L).
Total proteins were extracted, and Western blotting analysis was performed. The same blot was reprobed for β-actin as a loading control; hTERT: Human telomerase reverse transcriptase.
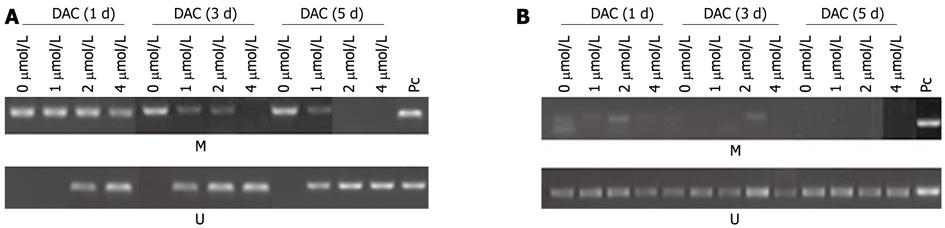
Figure 4 Methylation of telomerase reverse transcriptase promoter in 5-aza-2’-deoxycitidine-treated hepatocellular carcinoma cell lines SMMC-7721 (A) and HepG2 (B).
M: Methylation; U: Unmethylation; PC: Positive control; DAC: 5-aza-2’-deoxycitidine.
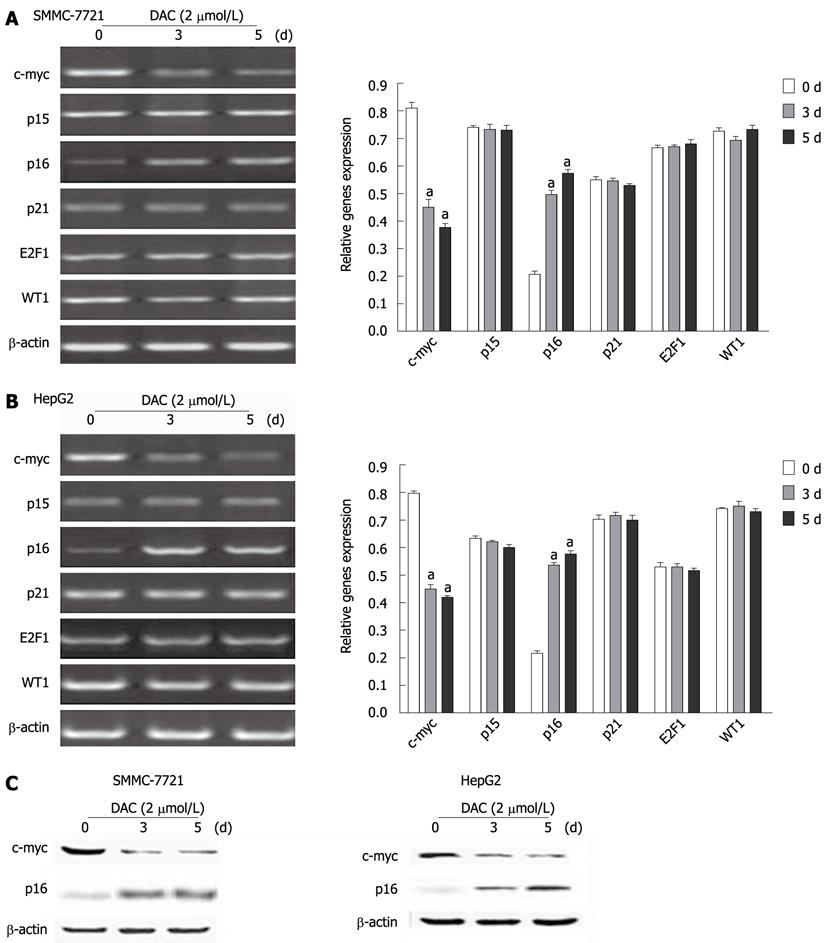
Figure 5 Effect of 5-aza-2’-deoxycitidine on expression of critical regulatory genes of telomerase reverse transcriptase transcription in hepatocellular carcinoma cell lines.
A, B: Cells were incubated with 2 μmol/L DAC for 3-5 d, and subjected to real-time reverse transcription-polymerase chain reaction assay, aP < 0.05 (by unpaired Student’s t test); C: Western blotting analysis of c-myc and p16 in hepatocellular carcinoma cell lines by DAC. The same blot was reprobed for β-actin as a loading control. DAC: 5-aza-2’-deoxycitidine.
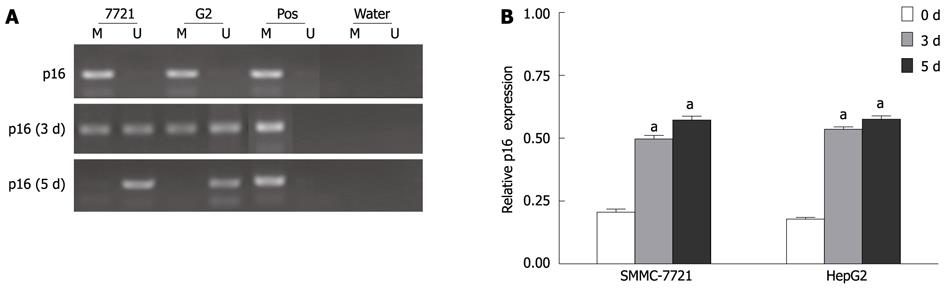
Figure 6 Demethylation of p16 promoter region and reactivation of p16 expression after 5-aza-2’-deoxycitidine treatment.
Cells were incubated with 2 μmol/L DAC for 3-5 d and subjected to methylation-specific polymerase chain reaction (MSP) and real-time reverse transcription-polymerase chain reaction assay, respectively. M: Methylation; U: Unmethylation; Pos: Positive control. aP < 0.05 (by unpaired Student’s t test). DAC: 5-aza-2’-deoxycitidine.
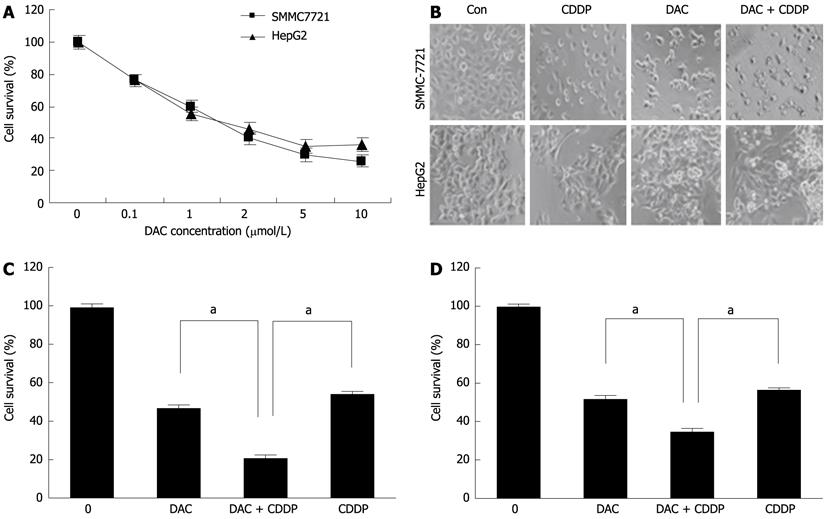
Figure 7 Sensitivity of SMMC-7721 and HepG2 cells to cisplatin enhanced by 5-aza-2’-deoxycitidine.
A: Effects of DAC on growth inhibition. The hepatocellular carcinoma cell lines SMMC-7721 and HepG2 were treated with various doses of DAC for 72 h. Cell viability was determined by cell proliferation assay kit; B: The morphology of the cells was captured under a light microscope; C, D: SMMC-7721 (C) and HepG2 cells (D) cells were treated with 2 μmol/L DAC, 20 μmol/L cisplatin, or in combination for 72 h. Cell viability was determined by MTT assays. DAC: 5-aza-2’-deoxycitidine; Con: Control; CDDP: Cisplatin.
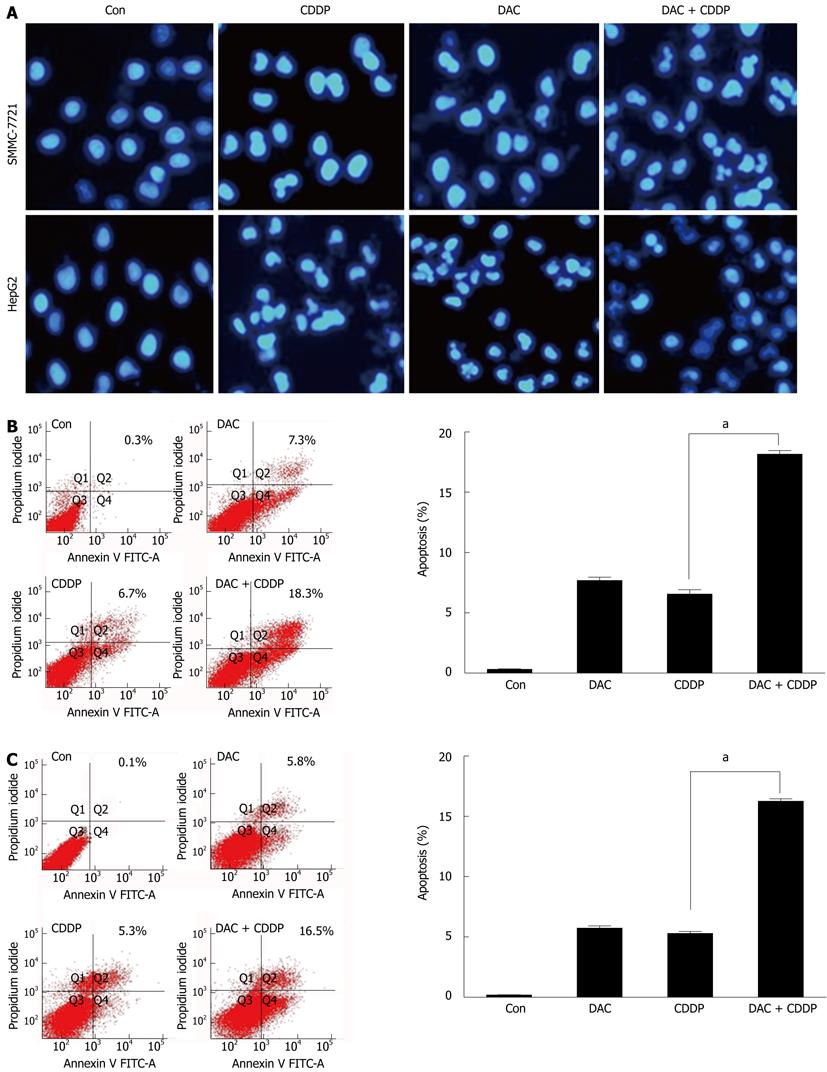
Figure 8 Apoptosis induced by 5-aza-2’-deoxycitidine.
SMMC-7721 and HepG2 cells were treated with 2 μmol/L DAC, 20 μmol/L cisplatin, or in combination for 72 h. A: The fluorescent microscopic pictures of apoptotic cells were captured by 4’,6-diamidino-2-phenylindole staining of the condensed and fragmented nuclei; B, C: Annexin V-fluorescein isothiocyanate (FITC) staining following facial action coding system analysis was shown for SMMC-7721 (B) or HepG2 cells (C). DAC: 5-aza-2’-deoxycitidine; Con: Control; CDDP: Cisplatin.
- Citation: Tao SF, Zhang CS, Guo XL, Xu Y, Zhang SS, Song JR, Li R, Wu MC, Wei LX. Anti-tumor effect of 5-aza-2'-deoxycytidine by inhibiting telomerase activity in hepatocellular carcinoma cells. World J Gastroenterol 2012; 18(19): 2334-2343
- URL: https://www.wjgnet.com/1007-9327/full/v18/i19/2334.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i19.2334