Copyright
©2010 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 21, 2010; 16(47): 5925-5935
Published online Dec 21, 2010. doi: 10.3748/wjg.v16.i47.5925
Published online Dec 21, 2010. doi: 10.3748/wjg.v16.i47.5925
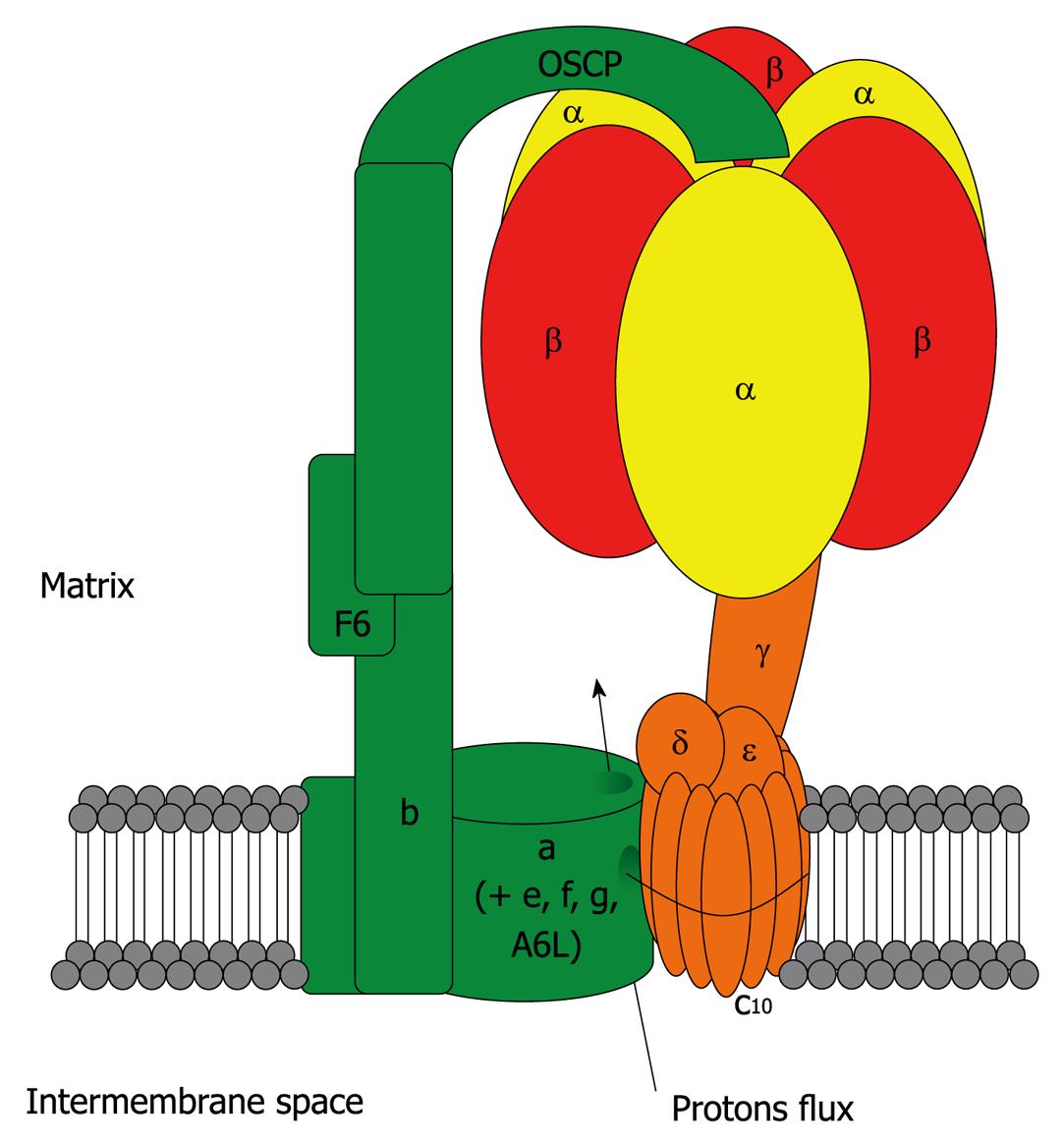
Figure 1 Mitochondrial ATP synthase.
In eukaryotic cells, F1FO ATP synthase is a complex molecular motor composed of 16 unique subunits (α, β and c being present in multiple copies in the whole complex). It comprises the activity-bearing subunits (three αβ heterodimers), a rotor (in orange: subunits γ, δ, ε and the c ring) and a stator (in green: subunits a, e, f, g, A6L, b, F6, d and OSCP) which holds the αβ heterodimers. It is organized into two major domains: a soluble F1 domain (α, β, γ, δ, ε) and a membrane-associated FO domain. The F1 domain bears the catalytic activity while the FO domain is a proton channel. The FO domain uses the proton gradient created by the four other complexes of the respiratory chain to drive the rotation of the central stalk, which will alternatively change the conformation of the three αβ heterodimers, leading to the synthesis of ATP from ADP and Pi. In the case of the eukaryotic ATP synthase, the translocation of 10 protons through the c ring will lead to a full turn, and the synthesis of 3 ATP molecules. In the absence of a proton gradient, or if the F1 domain is isolated from the FO domain, the enzyme behaves as an ATP hydrolase.
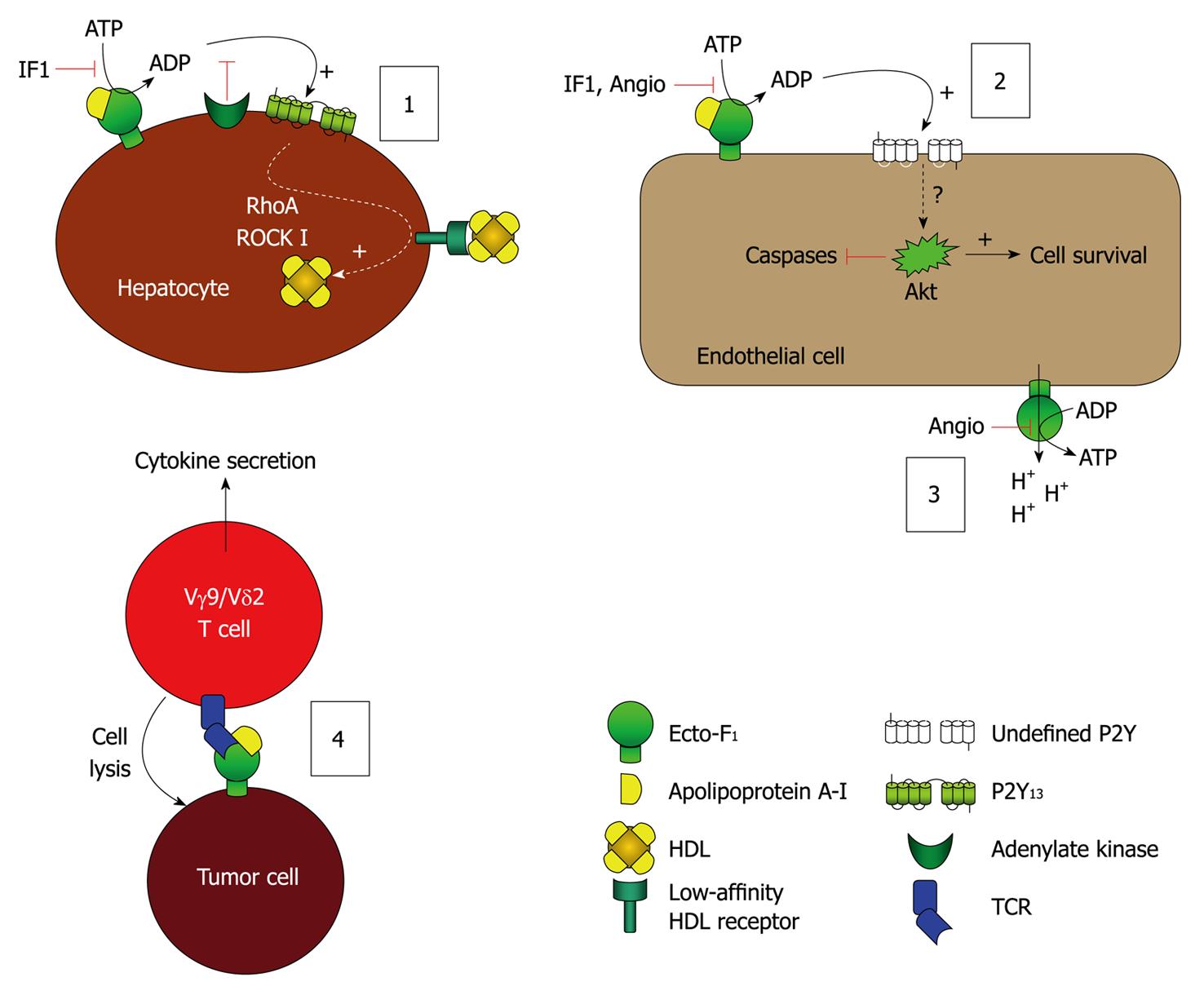
Figure 2 Model of the leading hypotheses regarding the distinct cellular events mediated by ecto-F1-ATPase.
(1) Model of F1-ATPase/P2Y13-mediated high density lipoproteins (HDL) endocytosis by hepatocytes. ApoA-I binding to ecto-F1-ATPase stimulates extracellular ATP hydrolysis, and the ADP generated selectively activates the nucleotide receptor P2Y13 and subsequent RhoA/ROCK I signaling that controls holo-HDL particle endocytosis. Conversely IF1 inhibitor which inhibits ecto-F1-ATPase activity or adenylate kinase (AK) activity which consumes ADP generated upon activation of ecto-F1-ATPase, downregulates holo-HDL particle endocytosis. Thus, HDL endocytosis depends on the balance between the AK activity that removes extracellular ADP and the ecto-F1-ATPase that synthesizes ADP. Activation of ADP synthesis by apoA-I unbalances the pathway and increases HDL endocytosis when required; (2), (3): Model of F1-ATPase-mediated endothelial cell protection. (2): Hydrolytic activity of ecto-F1-ATPase expressed on endothelial cells can be increased by apoA-I, stimulating cell proliferation. Conversely, inhibition by IF1, angiostatin increases apoptosis and blocks proliferation both at the basal level and after stimulation by apoA-I. The downstream receptors and intracellular signaling are still not completely characterized, but ADP-sensitive receptors could be involved; (3): The anti-angiogenic activity of angiostatin is attributed to its ability to inhibit ATP production by ecto-F1-ATPase, thus eliminating a significant source of energy and reducing proliferation. It is also proposed that this inhibition prevents the extrusion of protons by ecto-F1-ATPase, thus lowering intracellular pH and hampering cell survival; and (4) Model of F1-ATPase-mediated tumor cell recognition by Vγ9/Vδ2 T lymphocytes. Ecto-F1-ATPase is found at the surface of tumor cells and plays a role in the presentation of adenylated derivatives of small phosphoantigens, which are classical inducers of cell lysis by Vγ9/Vδ2 T lymphocytes. Recognition of ecto-F1-ATPase by the γ9/δ2 TCR is stimulated by apoA-I and requires a low expression of class I HLA-antigens, a situation commonly found in tumor cells.
- Citation: Vantourout P, Radojkovic C, Lichtenstein L, Pons V, Champagne E, Martinez LO. Ecto-F1-ATPase: A moonlighting protein complex and an unexpected apoA-I receptor. World J Gastroenterol 2010; 16(47): 5925-5935
- URL: https://www.wjgnet.com/1007-9327/full/v16/i47/5925.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i47.5925