Copyright
©2009 The WJG Press and Baishideng.
World J Gastroenterol. Jan 21, 2009; 15(3): 289-299
Published online Jan 21, 2009. doi: 10.3748/wjg.15.289
Published online Jan 21, 2009. doi: 10.3748/wjg.15.289
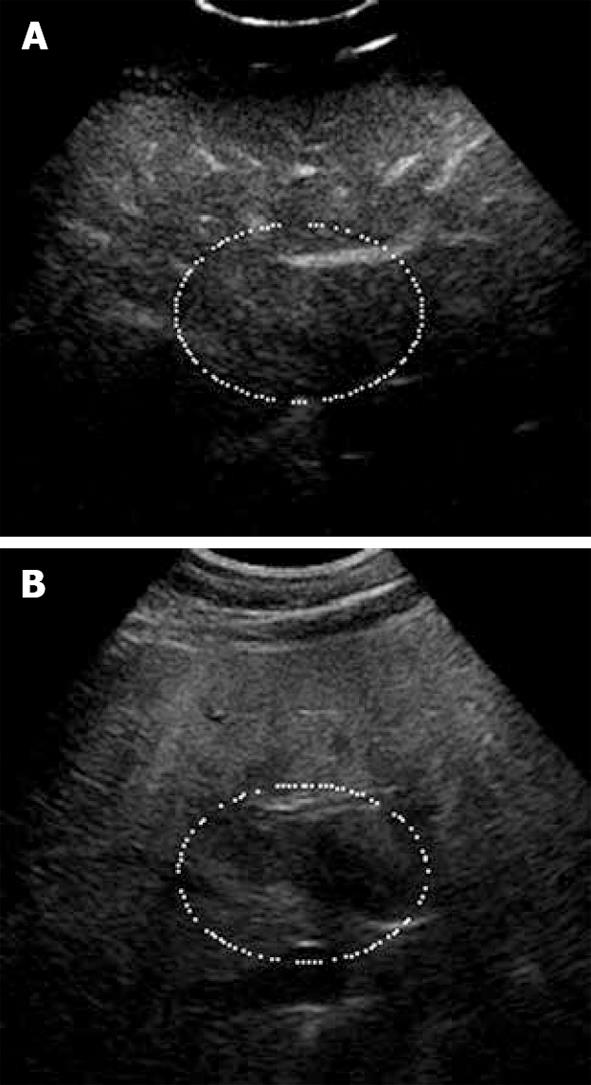
Figure 1 Focal fat sparing shown as iso-echogenicity as adjacent liver parenchyma.
A: Late phase of CE-US; B: Hypo-echoic area on the fundamental mode.
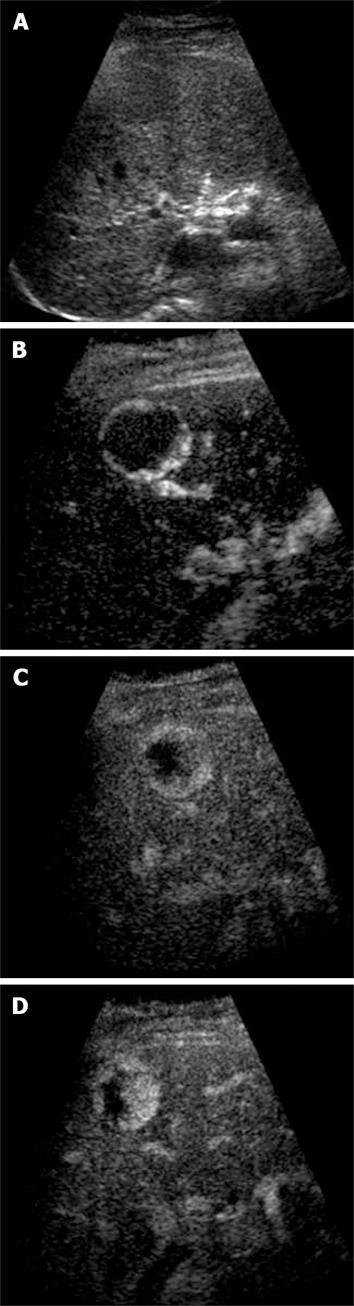
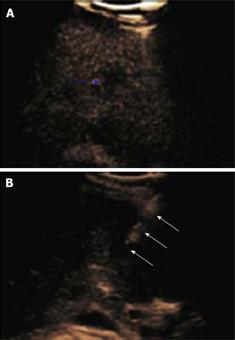
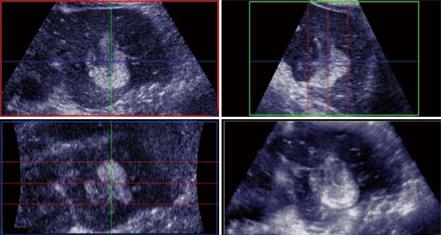
Figure 2 Haemangioma.
A: Baseline focal hypo-echoic lesion; B: Arterial phase showing peripheral rim enhancement; C: Portal venous phase showing peripheral globular rim enhancement; D: Late phase showing progressive centripetal filling-in which is characteristic for hemangioma.
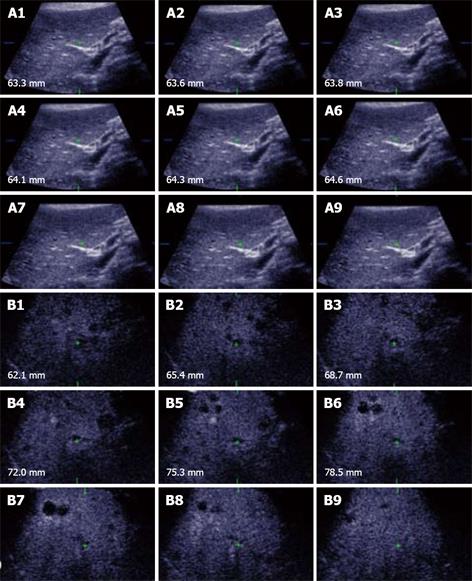
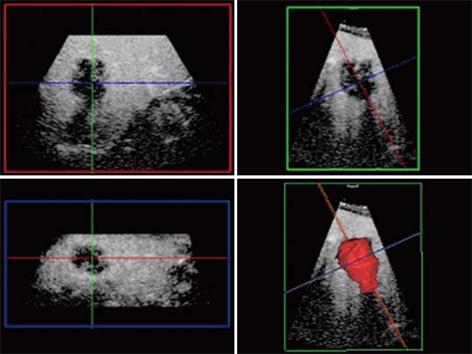
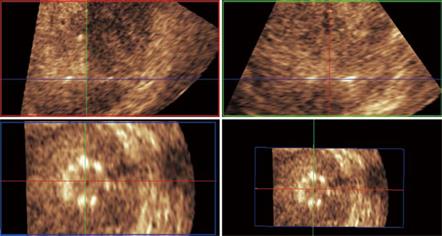
Figure 3 Unenhanced ultrasound and contrast enhanced 3D US.
A: Unenhanced Fundamental mode ultrasound showing apparently normal liver; B: Contrast enhanced 3D US displayed as axial scans showing numerous occult metastases appearing as filling defects in the late phase.
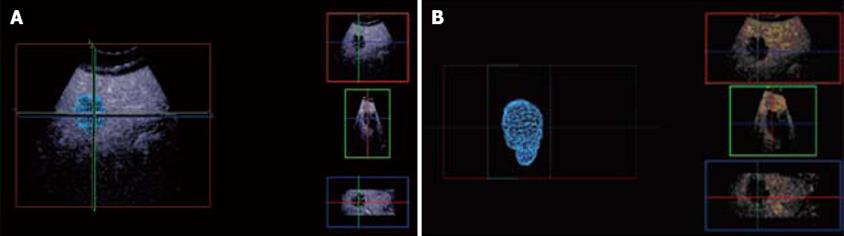
Figure 4 Multi-planar reconstruction.
A: Tumor appears to be spherical in tumor modeling; B: Background liver subtraction shows geometry of the tumor model and long axis.
Figure 5 Side-by-side screen.
Blue arrow points to small occult metastasis only seen on the CE-US non-linear imaging mode (A), whilst the open arrows point to the RFA needle electrode trajectory towards the occult metastasis, which is only visualized on the fundamental US scan (B).
Figure 6 RFA needle electrode.
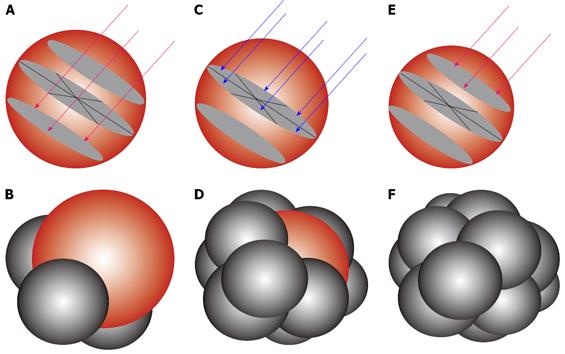
Modeling to show the placement of the RFA needle electrodes to enable 12 overlapping spheres to give an equivalent of 7.5 cm diameter target sphere. (A, B) lower pole ablation with 3 RFA needle electrodes (red arrows) to create 3 overlapping ablation spheres (C, D) middle row ablation with 6 RFA needle electrodes (blue arrows) to create 6 overlapping ablation spheres and (E, F) upper pole ablation with retraction of the first 3 RFA electrodes to complete the target sphere of 7.5 cm.
Figure 7 RFA needle electrode with expandable antennas.
Figure 8 Multi-planar reconstruction.
RFA Needle electrode placement along the long axis of the tumour mass which correspond to the planned “Red” axial plane bisecting the perpendicular the “Green” sagittal plane with reference to the probe position.
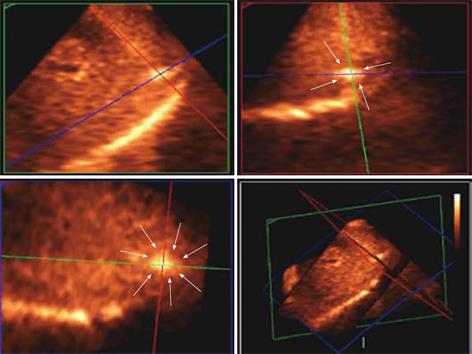
Figure 9 Matrix probe.
3D US showing the hyper-echoic needle electrode tip and its spatial position in the tumour (white arrows delineates the tumour margin) in the subcapsular area and is clearly depicted in 3 orthogonal 2D images confirming the tip being in the center of the tumour.
Figure 10 The 3 orthogonal planes showing the RFA needle electrodes with the multiple antennas deployed and clearly visualised on the (Blue: left lower quadrant) elevation plane following alignment of the Green and Red planes along the axis of the RFA needle electrode.
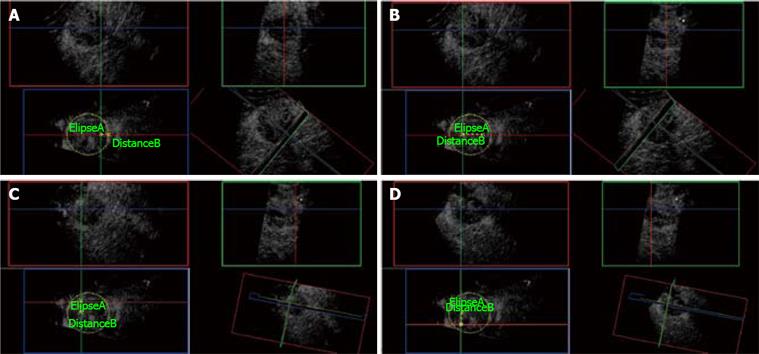
Figure 11 3D CE-US.
A: Ellipse marks the tumor border at its maximal diameter (5 cm). First needle electrode insertion is planned from the elevation plane (Blue box) at 1 cm from the edge of the tumor. Green and Red orthogonal planes mark the trajectory of the needle electrode. B: Distance B measures 2.5 cm from the first needle electrode insertion and plans the plane (Green) of insertion for the next two needle electrodes. C: The second needle electrode is placed at the Green and Red planes intersection measuring 1.5 cm superior to the last position. D: Third needle insertion is placed at the Red and Green plane intersection, 3 cm below the second needle electrode as shown on the Blue plane.
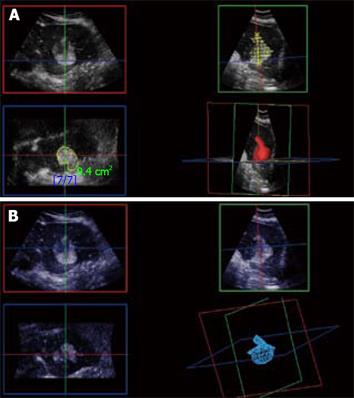
Figure 12 3D CE-US.
Arterial phase 3D acquisition showing recurrent enhancing HCC in 3 orthogonal planes and the volume rendering analysis (left lower quadrant).
Figure 13 3D reconstruction of local recurrence geometry in the planning for further ablation.
A: With normal liver background; B: Without normal liver background.
- Citation: Leen E, Kumar S, Khan SA, Low G, Ong KO, Tait P, Averkiou M. Contrast-enhanced 3D ultrasound in the radiofrequency ablation of liver tumors. World J Gastroenterol 2009; 15(3): 289-299
- URL: https://www.wjgnet.com/1007-9327/full/v15/i3/289.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.289