Copyright
©2008 The WJG Press and Baishideng.
World J Gastroenterol. Dec 7, 2008; 14(45): 6893-6901
Published online Dec 7, 2008. doi: 10.3748/wjg.14.6893
Published online Dec 7, 2008. doi: 10.3748/wjg.14.6893
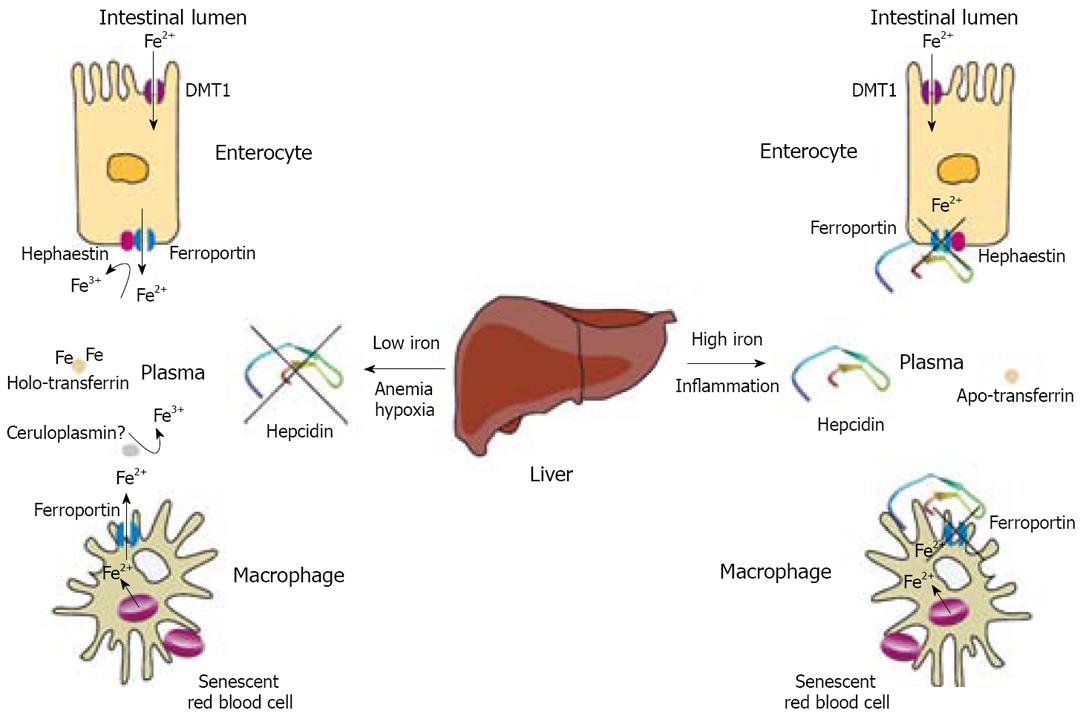
Figure 1 Regulation of iron efflux from enterocytes and macrophages by hepcidin.
Duodenal enterocytes absorb dietary iron via DMT1 and reticuloendothelial macrophages phagocytose iron-loaded senescent red blood cells. Both cell types release ferrous iron (Fe2+) into plasma via ferroportin, which is incorporated into transferrin following oxidation into the ferric form (Fe3+) via hephaestin or ceruloplasmin. The secretion of the iron-regulatory hormone hepcidin from the liver in response to high body iron stores or inflammatory signals results in internalization and degradation of ferroportin, and retention of iron within enterocytes and macrophages. A decrease in body iron stores, a requirement of iron for erythropoiesis, or hypoxia, inhibit hepcidin expression, permitting dietary iron absorption by enterocytes and iron release from macrophages.
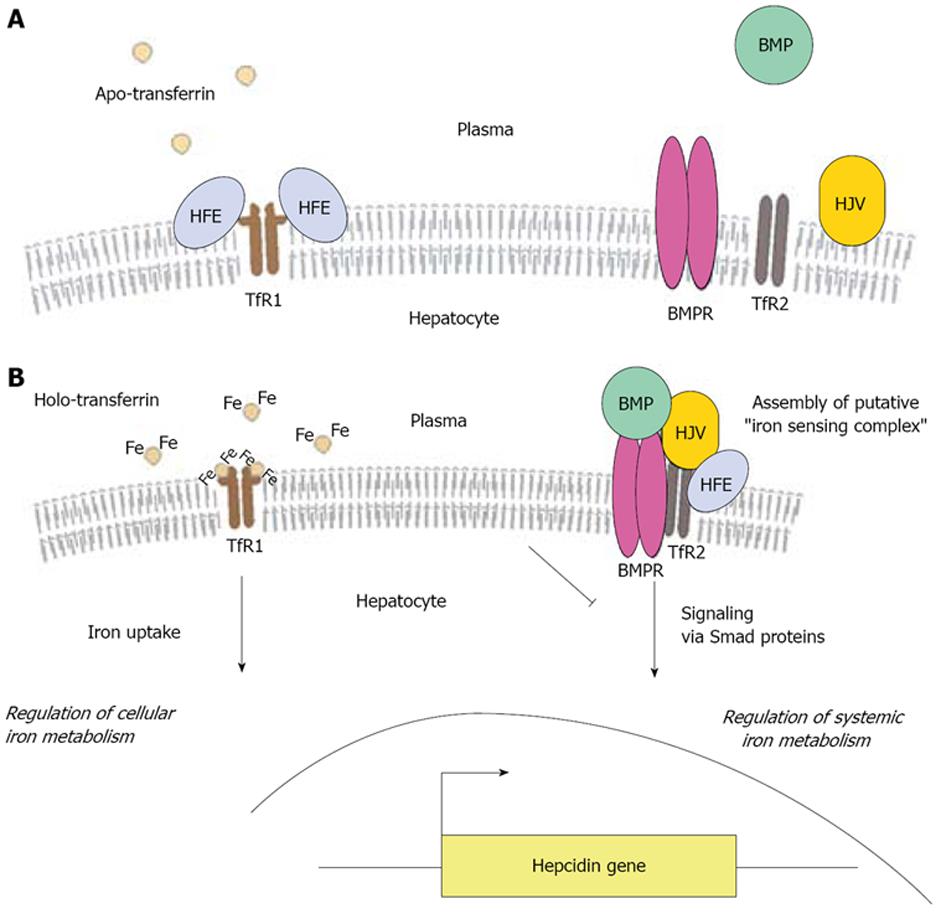
Figure 2 A model for HFE-me-diated signaling to hepcidin in hepatocytes.
A: At low plasma iron concentration, HFE is bound to TfR1 and other proteins involved in signaling to hepcidin remain silent; B: An increase in plasma iron levels results in displacement of HFE from TfR1, followed by iron uptake. This triggers the assembly of a putative “iron-sensing” complex, comprising of HFE, TfR2, BMPs (such as BMP-2, BMP-4 and BMP-9) and their receptor BMPR, and Hjv, which mediates signaling to activate hepcidin transcription via Smad proteins. Thus, the hepatocyte integrates signals for regulation of iron metabolism at the cellular and systemic level.
- Citation: Pantopoulos K. Function of the hemochromatosis protein HFE: Lessons from animal models. World J Gastroenterol 2008; 14(45): 6893-6901
- URL: https://www.wjgnet.com/1007-9327/full/v14/i45/6893.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6893